Abstract
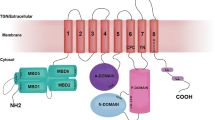
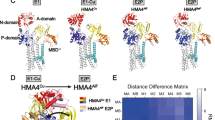
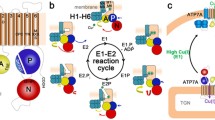
The Menkes copper-translocating P-type ATPase (ATP7A; MNK) is a ubiquitous protein that regulates the absorption of copper in the gastrointestinal tract. Inside cells the protein has a dual function: it delivers copper to cuproenzymes in the Golgi compartment and effluxes excess copper. The latter property is achieved through copper-dependent vesicular trafficking of the Menkes protein to the plasma membrane of the cell. The trafficking mechanism and catalytic activity combine to facilitate absorption and intercellular transport of copper. The mechanism of catalysis and copper-dependent trafficking of the Menkes protein are the subjects of this review. Menkes disease, a systemic copper deficiency disorder, is caused by mutations in the gene encoding the Menkes protein. The effect of these mutations on the catalytic cycle and the cell biology of the Menkes protein, as well as predictions of the effect of particular mutant MNKs on observed Menkes disease symptoms will also be discussed.
Similar content being viewed by others
REFERENCES
Bissig, K. D., Wunderli-Ye, H., Duda, P. W., and Solioz, M. (2001). Biochem. J. 357, 217–223.
Camakaris, J., Danks, D. M., Ackland, L., Cartwright, E., Borger, P., and Cotton, R., G. (1980). Biochem. Genet. 18, 117–131.
Camakaris, J., Petris, M. J., Bailey, L., Shen, P., Lockhart, P., Glover, T. W., Barcroft, C., Patton, J., and Mercer, J. F. (1995). Hum. Mol. Genet. 4, 2117–2123.
Camakaris, J., Voskoboinik, I., and Mercer, J. F. (1999). Biochem. Biophys. Res. Commun. 261, 225–232.
Chelly, J., Tumer, Z., Tonnesen, T., Petterson, A., Ishikawa Brush, Y., Tommerup, N., Horn, N., and Monaco, A. P. (1993). Nat. Genet. 3, 14–19.
Dagenais, S. L., Adam, A. N., Innis, J. W., and Glover, T. W. (2001). Am. J. Hum. Genet. 69, 420–427.
Daly, S. E., Lane, L. K., and Blostein, R. (1996). J. Biol. Chem. 271, 23683–23689.
Danks, D. M. (1995). In: The Metabolic Basis of Inherited Disease (Eds: Scriver, C. R., Beaudet, A. L., Sly, W. V., and Valle, D.). McGraw-Hill, New York, p. 2211–2235.
Fan, B., Grass, G., Rensing, C., and Rosen, B. P. (2001). Biochem. Biophys. Res. Commun. 286, 414–418.
Forbes J. R., and Cox, D. W. (2000). Hum. Mol. Genet. 9, 1927–1935.
Forbes, J. R., Hsi, G., and Cox, D.W. (1999). J. Biol. Chem. 274, 12408–12413.
Francis, M. J., Jones, E. E., Levy, E. R., Martin, R. L., Ponnambalam, S., and Monaco, A. P. (1999). J. Cell. Sci. 112, 1721–1732.
Francis, M. J., Jones, E. E., Levy, E. R., Ponnambalam, S., Chelly, J., and Monaco, A. P. (1998). Hum. Mol. Genet. 7, 1245–1252.
Fu, D., Beeler, T. J., and Dunn, T. M. (1995). Yeast 11, 283–292.
Goodyer, I. D., Jones, E. E., Monaco, A. P., and Francis, M. J. (1999). Hum. Mol. Genet. 8, 1473–1478.
Gu, Y. H., Kodama, H., Murata, Y., Mochizuki, D., Yanagawa, Y., Ushijima, H., Shiba, T., and Lee, C. C. (2001). Am. J. Med. Genet. 99, 217–222.
Hamza, I., Faisst, A., Prohaska, J., Chen, J., Gruss, P., and Gitlin, J. D. (2001). Proc. Natl. Acad. Sci. U. S. A. 98, 6848–6852.
Iida, M., Terada, K., Sambongi, Y., Wakabayashi, T., Miura, N., Koyama, K., Futai, M., and Sugiyama, T. (1998). FEBS Lett. 428, 281–285.
Irani, A. N., Malhi, H., Slehria, S., Gorla, G. R., Volenberg, I., Schilsky, M. L., and Gupta, S. (2001). Mol. Ther. 3, 302–309.
Kaler, S. G. (1998). Am. J. Clin. Nutr. 67(Suppl.), 1029–1034S.
Kaler, S. G., Das, S., Levinson, B., Goldstein, D. S., Holmes, C. S., Patronas, N. J., Packman, S., and Gahl, W. A. (1996). Biochem. Mol. Med. 57, 37–46.
Larin, D., Mekios, C., Das, K., Ross, B., Yang, A. S., and Gilliam, T. C. (1999). J. Biol. Chem. 274, 28497–28504.
Linder, M. C., and Hazegh Azam, M. (1996). Am. J. Clin. Nutr. 63, 797S–811S.
Lutsenko, S., Petrukhin, K., Cooper, M. J., Gilliam, C. T., and Kaplan, J. H. (1997). J. Biol. Chem. 272, 18939–18944.
Mercer, J. F., Livingston, J., Hall, B., Paynter, J. A., Begy, C., Chandrasekharappa, S., Lockhart, P., Grimes, A., Bhave, M., Siemieniak, D., and Glover, T. W. (1993). Nat. Genet. 3, 20–25.
Mitra, B., and Sharma, R. (2001). Biochemistry 40, 7694–7699.
Moller, L. B., Tumer, Z., Lund, C., Petersen, C., Cole, T., Hanusch, R., Seidel, J., Jensen, L. R., and Horn, N. (2000). Am. J. Hum. Genet. 66, 1211–1220.
Mollman, J. E., and Pleasure, D. E. (1980). J. Biol. Chem. 255, 569–574.
Oh, W. J., Kim, E. K., Ko, J. H., Yoo, S. H., Hahn, S. H., and Yoo, O. J. (2002). Eur. J. Biochem. 269, 2151–2161.
Oh, W. J., Kim, E. K., Park, K. D., Hahn, S. H., and Yoo, O. J. (1999). Biochem. Biophys. Res. Commun. 259, 206–211.
Payne, A. S., and Gitlin, J. D. (1998). J. Biol. Chem. 273, 3765–3770.
Paynter, J. A., Grimes, A., Lockhart, P., and Mercer, J. F. (1994). FEBS Lett. 351, 186–190.
Petris, M. J., Camakaris, J., Greenough, M., LaFontaine, S., and Mercer, J. F. B. (1998). Hum. Mol. Genet. 7, 2063–2071.
Petris, M. J., Camakaris, J., Voskoboinik, I., Kim, B.-E., Smith, K., and Mercer, J. F. (2002). In 11th International Symposium on Trace Elements in Man and Medicine, June 2-6, Berkeley, CA, p. 108.
Petris, M. J., and Mercer, J. F. (1999). Hum. Mol. Genet. 8, 2107–2115.
Petris, M. J., Mercer, J. F., Culvenor, J. G., Lockhart, P., Gleeson, P. A., and Camakaris, J. (1996). EMBO J. 15, 6084–6095.
Rad, M. R., Kirchrath, L., and Hollenberg, C. P. (1994). Yeast 10, 1217–1225.
Rae, T. D., Schmidt, P. J., Pufahl, R. A., Culotta, V.C., and O'Halloran, T. V. (1999). Science 284, 805–808.
Rensing, C., Fan, B., Sharma, R., Mitra, B., and Rosen, B. P. (2000). Proc. Natl. Acad. Sci. U.S.A. 97, 652–656.
Rensing, C., Mitra, B., and Rosen, B. P. (1997). Proc. Natl. Acad. Sci. U.S.A. 94, 14326–14331.
Saari, J. T. (2000). Can. J. Physiol. Pharmacol. 78, 848–855.
Schilsky, M. L. (1994). Hepatology 20, 529–533.
Seidel, J., Birk Moller, L., Mentzel, H.-J., Kauf, E., Vogt, S., Patzer, S., Wollina, U., Zintl, F., and Horn, N. (2001). Cell. Mol. Biol. 47, 141–148.
Shiraishi, E., Inouhe, M., Joho, M., and Tohoyama, H. (2000). Curr. Genet. 37, 79–86.
Strausak, D., La Fontaine, S., Hill, J., Firth, S. D., Lockhart, P. J., and Mercer, J. F. (1999). J. Biol. Chem. 274, 11170–11177.
Tsai, K. J., and Linet, A. L. (1993). Arch. Biochem. Biophys. 305, 267–270.
Tsivkovskii, R., Eisses, J. F., Kaplan, J. H., and Lutsenko, S. (2002). J. Biol. Chem. 277, 976–983.
Tsivkovskii, R., MacArthur, B. C., and Lutsenko, S. (2001). J. Biol. Chem. 276, 2234–2242.
Tumer, Z., Lund, C., Tolshave, J., Vural, B., Tonnesen, T., and Horn, N. (1997). Am. J. Hum. Genet. 60, 63–71.
Tumer, Z., Moller, L. B., and Horn, N. (1999). Adv. Exp. Med. Biol. 448, 83–95.
Vanderwerf, S. M., Cooper, M. J., Stetsenko, I. V., and Lutsenko, S. (2001). J. Biol. Chem. 276, 36289–36294.
Voskoboinik, I., Brooks, H., Smith, S., Shen, P., and Camakaris, J. (1998). FEBS Lett. 435, 178–182 Molecular Properties of the Menkes Copper P-Type ATPase 371
Voskoboinik, I., Greenough, M., La Fontaine, S., Mercer, J. F., and Camakaris, J. (2001a). Biochem. Biophys. Res. Commun. 281, 966–970.
Voskoboinik, I., Mar, J., Strausak, D., and Camakaris, J. (2001b). J. Biol. Chem. 276, 28620–28627.
Voskoboinik, I., Strausak, D., Greenough, M., Brooks, H., Petris, M., Smith, S., Mercer, J. F., and Camakaris, J. (1999). J. Biol. Chem. 274, 22008–22012.
Vulpe, C., Levinson, B., Whitney, S., Packman, S., and Gitschier, J. (1993). Nat. Genet. 3, 7–13.
Yoshida, Y., Tokusashi, Y., Lee, G. H., and Ogawa, K. (1996). Gastroenterology 111, 1654–1660.
Yuan, D. S., Dancis, A., and Klausner, R. D. (1997). J. Biol. Chem. 272, 25787–25793.
Yuan, D. S., Stearman, R., Dancis, A., Dunn, T., Beeler, T., and Klausner, R. D. (1995). Proc. Natl. Acad. Sci. U.S.A. 92, 2632–2636.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Voskoboinik, I., Camakaris, J. Menkes Copper-Translocating P-type ATPase (ATP7A): Biochemical and Cell Biology Properties, and Role in Menkes Disease. J Bioenerg Biomembr 34, 363–371 (2002). https://doi.org/10.1023/A:1021250003104
Issue Date:
DOI: https://doi.org/10.1023/A:1021250003104