Abstract
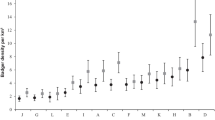
We examined the relative importance of ecological parameters—habitat productivity and seasonality—and group history—episodic predation, disease, and sudden habitat deterioration—to explain variation in the density and group structure of howlers (Alouatta spp.). We use data from a census of Guanacaste National Park, Costa Rica, and a literature review characterizing 80 howler populations. In Guanacaste National Park both habitat type and degree of protection affect howler density and group structure. Howlers were found at the highest density and in the largest groups in areas of semievergreen forest, which ecological sampling indicates have the most consistent level of food production. Differences in density between the sector of the park that first received protected status and more recently protected areas may be due partially to the degree of protection the areas received. We test the prediction that howler density and group structure would be influenced by habitat productivity as indexed by rainfall. Average group size and sex ratios differ among species, but female-to-immature ratios do not. Considering all censuses at one site to be independent, there are significant interspecific differences in density, with Alouatta pigra occurring at lower densities than the other species. In spite of such variability, there is no relationship between annual rainfall and howler density, and rainfall had a variable effect on group size depending on the level of independence that was considered. While such ecological comparisons are unrefined, e.g., rainfall must be used as a surrogate for habitat production, the fact that so few relationships were documented suggests that factors other than the ecological factors considered here are responsible for the observed differences in population characteristics. We suggest that much of the variability in howler population characteristics is related to events occurring in the recent history of the groups, such as habitat alteration, hunting, food tree crop failure, and disease.
Similar content being viewed by others
REFERENCES
Altmann, S. A. (1959). Field observations on a howling monkey society. J. Mammal. 40:317–330.
Altmann, S. A., and Altmann, J. (1979). Demographic constraints on behaviour and social organization. In Bernstein, I. S., and Smith, E. O. (eds.), Primate Ecology and Human Origins, Garland Press, New York, pp. 47–63.
Baldwin, J. D., and Baldwin, J. I. (1972). Population density and use of space in howling monkeys (Alouatta villosa) in southwestern Panama. Primates 13:371–379.
Baldwin, J. D., and Baldwin, J. I. (1976). Primate populations in Chiriqui, Panama. In Thorington, R. W., and Heltne, P. N. (eds.), Neotropical Primates. Field Studies and Conservation, National Academy of Sciences, Washington, DC, pp. 20–31.
Bernstein, I. S. (1964). A field study of the activities of howler monkeys. Anim. Behav. 12:92–97.
Bolin, I. (1981). Male parental behaviour in black howler monkeys (Alouatta palliata pigra) in Belize and Guatemala. Primates 22: 349–360.
Braza, A. A., Alvarez, F., and Azcarate, T. (1981). Behaviour of the red howler monkey (Alouatta seniculus) in the llanos of Venezuela. Primates 22: 459–473.
Butynski, T. T. (1990). Comparative ecology of blue monkeys (Cercopithecus mitis) in high-and low-density subpopulations. Ecol. Monogr. 60: 1–26.
Carpenter, C. R. (1934). A field study of the behaviour and social relations of howling monkeys. Comp. Psychol. Monogr. 10: 1–168.
Carpenter, C. R. (1962). Field studies of a primate population. In Bliss, E. L. (ed.), Roots of Behavior, Harper and Row, New York, pp. 286–294.
Chapman, C. A. (1987). Flexibility in diets of three species of Costa Rican primates. Folia Primatol. 49: 90–105.
Chapman, C. A. (1988). Patterns of foraging and range use by three species of neotropical primates. Primates 29: 177–194.
Chapman, C. A. (1989). Ecological constraints on group size in three species of neotropical primates. Folia Primatol. 73: 1–9.
Chapman, C. A., and Chapman, L. J. (1990). Density and growth rate of some tropical dry forest trees: Comparisons between successional forest types. Bull. Torr. Bot. Club 117: 226–231.
Chapman, C. A., and Fedigan, L. M. (1990). Dietary differences between neighboring cebus monkey groups: Local tradition or responses to food availability. Folia Primatol. 54: 177–186.
Chapman, C. A., Chapman, L. J., and Glander, K. E. (1989). Primate populations in northwestern Costa Rica: Potential for recovery. Primate Conserv. 10: 37–44.
Chapman, C. A., Chapman, L. J., Wrangham, R. W., Isabirye-Basuta, G., and Ben-David, K. (1997). Spatial and temporal variation in the structure of a tropical forest. Afr. J. Ecol. 35: 287–302.
Chivers, D. J. (1969). On the daily behaviour and spacing of howling monkey groups. Folia Primatol. 10: 48–102.
Clarke, M. R., Zucker, E. L., and Scott, N. J. (1986). Population trends of the mantled howler groups of La Pacifica, Guanacaste, Costa Rica. Am. J. Primatol. 11: 79–88.
Coehlo, A. M., Coehlo, L. S., Bramblett, C. A., Bramblett, S. S., and Quick, L. B. (1976). Ecology, population characteristics and sympatric associations in primates: A bioenergetic analysis of howler and spider monkeys in Tikal. Yrbk. Phys. Anthropol. 20: 96–135.
Collias, N., and Southwick, C. (1952). A field study of population density and social organization in howling monkeys. Proc. Am. Phil. Soc. 96: 143–156.
Crockett, C. M. (1984). Emigration by female red howler monkeys and the case for female competition. In Small, M. F. (ed.), Female Primates: Studies by Women Primatologists, Alan R. Liss, New York, pp. 159–173.
Crockett, C. M. (1985). Population studies of red howler monkeys (Alouatta seniculus). Nat. Geogr. Res. 1: 264–273.
Crockett, C. M. (1996). The relation between red howler troop size and population growth in two habitats. In Norconk, M., Rosenberger, A., and Garber, P. (eds.), Adaptive Radiations of Neotropical Primates, Plenum Press, New York, pp. 489–510.
Crockett, C. M. (1998). Conservation biology of the genus Alouatt. Int. J. Primatol. 19: 549–578.
Crockett, C. M., and Eisenberg, J. F. (1987). Howlers: Variation in group size and demography. In Smuts, B., Cheney, D., Seyfarth, R., Wrangham, R. W., and Struhsaker, T. T. (eds.), Primate Societies, Chicago University Press, Chicago, pp. 54–68.
Crockett, C. M., and Rudran, R. (1987a). Red howler monkey birth data. I. Seasonal variation. Am. J. Primatol. 13: 347–368.
Crockett, C. M., and Rudran, R. (1987b). Red howler monkey birth data. II. Interannual, habitat, and sex comparisons. Am. J. Primatol. 13: 369–384.
Davies, A. G. (1994). Colobine populations. In Davies, A. G., and Oates, J. F. (eds.), Colobine Monkeys: Their Ecology, Behaviour, and Evolution, Cambridge University Press, Cambridge, pp. 285–310.
Defler, T. R. (1981). The density of Alouatta seniculus in the eastern llanos of Colombia. Primates 22: 564–569.
Dittus, W. P. J. (1980). The social regulation of primate populations: A synthesis. In Lindburg, D. G. (ed.), The Macaques: Studies in Ecology, Behaviour and Evolution, Van Nostrand Reinhold, New York, pp. 263–286.
Dunbar, R. I. M. (1979). Population demography, social organization, and mating strategies. In Bernstein, I. S., and Smith, E. O. (eds.), Primate Ecology and Human Origins, Garland Press, New York, pp. 65–88.
Eisenberg, J. F. (1979). Habitat, economy, and society: Some correlations and hypotheses for the neotropical primates. In Bernstein, I. S., and Smith, E. O. (eds.), Primate Ecology and Human Origins, Garland Press, New York, pp. 215–262.
Estrada, A. (1982). Survey and census of howler monkeys (Alouatta palliata) in the rain forest of “Los Tuxtlas” Veracruz, Mexico. Am. J. Primatol. 2: 363–372.
Fedigan, L. M. (1987). Demographic trends in the Alouatta palliata and Cebus capucinus populations of Santa Rosa National Park, Costa Rica. In Else, J. G., and Lee, P. C. (eds.), Primate Ecology and Conservation, Cambridge University Press, London, pp. 285–293.
Fedigan, L. M., Fedigan, L., and Chapman, C. A. (1985). A census of Alouatta palliata and Cebus capucinus monkeys in Santa Rosa National Park, Costa Rica. Brenesia 23: 309–322.
Fishkind, A. S., and Susman, R. W. (1987). Preliminary survey of the primates of the Zona Protectora and La Selva Biological Station, northeast Costa Rica. Primate Conserv. 8: 63–66.
Foster, R. B. (1982). Famine on Barro Colorado Island. In Leigh, E. G., Rand, A. S., and Windsor, D. M. (eds.), The Ecology of a Tropical Forest, Smithsonian Institution Press, Washington, DC, pp. 201–212.
Frankie, G. W., Baker, H. G., and Opler, P. A. (1974). Comparative phenological studies of trees in tropical wet and dry forest in the lowlands of Costa Rica. J. Ecol. 62: 881–919.
Freese, C. (1976). Censusing Alouatta palliata and Cebus capucinus in Costa Rican dry forest. In Thorington, R. W., and Heltne, P. G. (eds.), Neotropical Primates: Field Studies and Conservation, National Academy of Sciences, Washington, DC, pp. 4–9.
Freese, C., Heltne, P. G., Castro, R. N., and Whitesides, G. (1982). Patterns and determinants of monkey densities in Peru and Bolivia, with notes on distribution. Int. J. Primatol. 3: 53–90.
Froehlich, J. W., Thorington, R. W., and Otis, J. S. (1981). The demography of howler monkeys (Alouatta palliata) on Barro Colorado Island, Panama. Int. J. Primatol. 2: 207–236.
Ganzhorn, J. U. (1992). Leaf chemistry and the biomass of folivorous primates in tropical forests: Test of a hypothesis. Oecologia 91: 540–547.
Gaulin, S. J. C., and Gaulin, C. K. (1982). Behavioral ecology of Alouatta seniculus in Andean cloud forest. Int. J. Primatol. 3: 1–32.
Gaulin, S. J. C., Knight, D. H., and Gaulin, C. K. (1980). Local variation in Alouatta group size and food availability on Barro Colorado Island. Biotropica 12: 137–143.
Glander, K. E. (1980). Reproduction and population growth in free-ranging mantled howling monkeys. Am. J. Phys. Anthropol. 53: 25–36.
Glander, K. E. (1992). Dispersal patterns in Costa Rican mantled howling monkeys. Int. J. Primatol. 13: 415–436.
Green, S. (1978). Primate censusing in northern Colombia: A comparison of two techniques. Primates 19: 537–550.
Hall, J. B. (1977). Forest types in Nigeria: An analysis of pre-exploitation forest enumeration. J. Ecol. 65: 187–199.
Hall, J. B., and Swaine, M. D. (1976). Classification and ecology of closed-canopy forest in Ghana. J. Ecol. 64: 913–951.
Hall, J. B., and Swaine, M. D. (1981). Distribution and Ecology of Vascular Plants in a Tropical Rain Forest, Dr. W. Junk, The Hague.
Hartshorn, G. S. (1983). Plants. In Janzen, D. H. (ed.), Costa Rican Natural History, University of Chicago Press, Chicago, pp. 118–157.
Heltne, P. G., Turner, D. C., and Scott, N. J. (1976). Comparisons of census data on Alouatta palliata from Costa Rica and Panama. In Thorington, R. W., and Heltne, P. G. (eds.), Neotropical Primates. Field Studies and Conservation, National Academy of Sciences, Washington, DC, pp. 10–19.
Horwich, R. H. (1983). Species status of the black howler monkey, Alouatta pigra, of Belize. Primates 24: 288–289.
Horwich, R. H., and Johnson, E. D. (1986). Geographical distribution of the black howler (Alouatta pigra) in Central America. Primates 27: 53–62.
Hubbell, S. P. (1979). Tree dispersion, abundance, and diversity in a tropical dry forest. Science 203: 1299–1309.
Isbell, L. (1990). Sudden short-term increase in mortality of vervet monkeys (Cercopithecus aethiops) due to leopard predation in Amboseli National Park, Kenya. Am. J. Primatol. 21: 41–52.
Izawa, K. (1976). Group sizes and compositions of monkeys in the upper Amazon basin. Primates 17: 367–399.
Janzen, D. H. (1975). Ecology of Plants in the Tropics, Edward Arnold, London.
Janzen, D. H. (1986). Guanacaste National Park, Tropical, Ecological, and Cultural Restoration, Editorial Universidad Estatal a Distancia, San Jose, Costa Rica.
Klein, L. L., and Klein, D. J. (1976). Population density, and regional distribution in La Macarena, Colombia. In Thorington, R. W., and Heltne, P. G. (eds.), Neotropical Primates: Field Studies and Conservation, National Academy of Sciences, Washington, DC, pp. 70–78.
Leighton, M., and Leighton, D. R. (1982). The relationship of size of feeding aggregate to size of food patch: Howler monkeys (Alouatta palliata) feeding in Trichilia cipo fruit trees on Barro Colorado Island. Biotropica 14: 81–90.
McKey, D. B. (1978). Soils, vegetation, and seed-eating by black colobus monkeys. In Montgomery, G. G. (ed.), The Ecology of Arboreal Folivores, Smithsonian Institution Press, Washington, DC, pp. 423–437.
Milton, K. (1980). The Foraging Strategy of Howler Monkeys: A Study in Primate Economics, Columbia University Press, New York.
Milton, K. (1982). Dietary quality and demographic regulation in a howler monkey population. In Leigh, E. G., Rand, A. S., and Windsor, D. M. (ed.). The Ecology of a Tropical Forest, Smithsonian Institution Press, Washington, DC, pp. 273–289.
Milton, K. (1996). Effects of bot fly (Alouattamyia baeri) parasitism on a free-ranging howler monkey (Alouatta palliata) population in Panama. J. Zool. Lond. 239: 39–63.
Milton, K., and Mittermeier, R. A. (1977). A brief survey of the primates of Coiba Island, Panama. Primates 18: 931–936.
Mittermeier, R. A. (1973). Group activity and population dynamics of the howler monkey on Barro Colorado Island. Primates 14: 1–19.
Murphy, P. G., and Lugo, A. E. (1986). Ecology of tropical dry forest. Annu. Rev. Ecol. Syst. 17: 67–88.
Neville, M. K. (1972). The population structure of red howler monkeys (Alouatta seniculus) in Trinidad and Venezuela. Folia Primatol. 17: 56–86.
Neville, M. K. (1976). The population and conservation of howler monkeys in Venezuela and Trinidad. In Thorington, R. W., and Heltne, P. G. (eds.), Neotropical Primates: Field Studies and Conservation, National Academy of Sciences, Washington, DC, pp. 101–109.
Oates, J. F. (1977). The guereza and its foods. In Clutton-Brock, T. T. (ed.), Primate Ecology, Academic Press, London, pp. 275–321.
Oates, J. F., Whitesides, G. H., Davies, A. G., Green, S. M., Dasilva, G. L., and Mole, S. (1990). Determinants of variation in tropical forest primate biomass: New evidence from West Africa. Ecology 71: 328–343.
Olupot, W. J., Chapman, C. A., Brown, C. H., and Waser, P. M. (1994). Mangabey (Cercocebus albigena) population density, group size, and ranging: A twenty-year comparison. Am. J. Primatol. 32: 197–205.
Peres, C. A. (1997a). Primate community structure at twenty western Amazonian flooded and unflooded forests. J. Trop. Ecol. 13: 381–405.
Peres, C. A. (1997b). Effects of habitat quality and hunting pressure on arboreal folivore density in Neotropical forests: A case study of howler monkeys (Alouatta spp.). Folia Primatol. 68: 199–222.
Pope, B. (1966). Population characteristics of howler monkeys (Alouatta caraya) in northern Argentina. Am. J. Phys. Anthropol. 24: 361–370.
Pope, B. (1968). Population characteristics. Biblio. Primatol. 7: 13–70.
Richards, P. W. (1996). The Tropical Rainforest, 2nd ed., Cambridge University Press, Cambridge.
Robin, L. E., and Bazilevich, R. (1967). Production and Mineral Cycling in Terrestrial Vegetation. Oliver and Boyd, London.
Rodriguez, M. A. R. (1985). Algunos aspectos sobre comportamiento, alimentacion y nivel de poblacion de los monos (Primates:Cebidae) en el Refugio De Fauna Silvestre Palo Verde (Guanacaste, Costa Rica). In Investigaciones Sobre Fauna Silvestre de Costa Rica, Editorial Universidad Estatal a Distancia, San Jose, Costa Rica.
Rudran, R. (1979). The demography and social mobility of a red howler (Alouatta seniculus) population in Venezuela. In Eisenberg, J. F. (ed.), Vertebrate Ecology in the Northern Neotropics, Smithsonian Institution Press, Washington, DC, pp. 107–126.
Schlichte, H. (1978). A preliminary report on the habitat utilization of a group of howler monkeys (Alouatta villosa pigra) in the National Park of Tikal, Guatemala. In Montomery, G. G. (ed.), The Ecology of Arboreal Folivores, Smithsonian Institution Press, Washington, DC, pp. 551–559.
Smith, C. C. (1977). Feeding behavior of social organization in howling monkeys. In Clutton-Brock, T. T. (ed.), Primate Ecology, Academic Press, London, pp. 97–126.
Struhsaker, T. (1978). Food habits of five monkey species in the Kibale Forest, Uganda. In Chivers, D. J., and Herbert, J. (ed.), Recent Advances in Primatology, Academic Press, New York, pp. 225–248.
Terborgh, J. (1983). Five New World Primates, Princeton University Press, Princeton, NJ.
Thorington, R. W., Ruiz, J. C., and Eisenberg, J. F. (1984). A study of black howler monkeys (Alouatta caraya) populations in northern Argentina. Am. J. Primatol. 6: 357–366.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chapman, C.A., Balcomb, S.R. Population Characteristics of Howlers: Ecological Conditions or Group History. International Journal of Primatology 19, 385–403 (1998). https://doi.org/10.1023/A:1020352220487
Issue Date:
DOI: https://doi.org/10.1023/A:1020352220487