Abstract
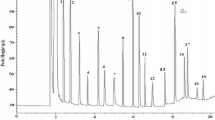
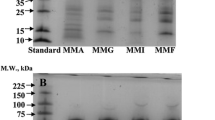
The storage proteins of the pili nut (Canarium ovatum, Engl.), quantitatively extracted using the modified Osborne protein fractionationscheme, revealed that the salt-soluble globulin was the main storage proteinwithin the kernel (i.e., >>60.3%). Further physicochemicalcharacterization of this aqueous soluble globulin revealed that it existed in an11S like form and was composed of two main subunits of 22,600 and31,600 Da. These subunits were found to be disulfide-linked (in a 1:1ratio) forming intermediary subunits (i.e., dimers) with a molecular weightof approximately 52,600 Da. The overall molecular weight of the 11Sglobulin was determined to be approximately 300,000 Da suggesting thatthe globulin possessed a dodecameric-like structure of 6 dimers for a totalof 12 subunits. Using differential scanning microcalorimetry, thedenaturation temperature of the globulin was shown to occur at89.3 °C. Overall, the pili nut 11S globulin was found to possessmany similar physicochemical properties to those of other 11S oilseedglobulins.
Similar content being viewed by others
References
Coronel RE, Zuno JC (1980a) Note: The correlation between some fruit characters of pili. Philip Agric 63: 163–165.
Coronel RE, Zuno JC (1980b) Note: Evaluation of fruit characters of some pili seedling trees in Calauan and Los Banos, Laguna. Philip Agric 63: 166–173.
Boquiren-Arribas L. Primer on Pili (Canarium ovatum Engl.), Published by DeMontano Foundation, Inc., Makati, Phil., Oct 1994.
Mohr E, Wichmann G (1987) Cultivation of Pili nut Canarium ovatum and the composition of fatty acids and triglycerides of oil. Fett Wiss Technol 89: 128–129.
American Association of Cereal Chemists (AACC) (1983) Approved Methods of the American Association of Cereal Chemists, 8th edn. St. Paul, MN: AACC, Inc.
Bio-Rad Laboratories Technical Bulletin (1989) LIT 89-0931, 1089.
Bhushan, R, Reddy K (1989) Reconstitution of conarchin from isolated subunits. Intl J Prot Pept Res 33: 313–316.
Fukushima D (1991a) Structures of plant storage proteins and their function. Food Rev Intl 7: 353–381.
Osborne TB (1924) The Vegetable Proteins. London, UK: Longmans Greens.
Lambert N, Yarwood JN (1992) Engineering legume seed storage proteins. In: Shewry PR, Gutteridge S (eds), Plant Protein Engineering. London, UK: Cambridge University Press, pp 167–187.
Marcone MF, Kakuda K, Yada RY (1998a) Salt-soluble seed globulins of various dicotyledonous and monocotyledonous plants. I. Isolation/purification and characterization. Food Chem 6261: 27–47.
Byers M, Miflin BJ, Smith SJ (1983) A quantitative comparison of the extraction of protein fractions from wheat grain by different solvents and of the polypeptide and amino acid composition of the alcohol-soluble proteins. J Sci Food Agric 34: 447–462.
Fu BX, Sapirstein HD (1996) Procedure for isolating monomeric protein and polymoric glutenin of wheat flour. Cereal Chem 73: 143–152.
Barba de la Rosa AP, Paredez-López O, Guoguen J (1992) Characterization of Amaranth globulins by ultracentrifugation and chromatographic techniques. J Agric Food Chem 40: 937–940.
Yamada T, Aibara S, Morita Y (1979) Dissociation-association behavior of arachin between dimeric and monomeric forms. Agric Biol Chem 43: 2549–2556.
Badley RA, Atkinson D, Hauser H, Oldani D, Green JP, Stubbs JM (1975) The structure, physical and chemical properties of the soy bean protein glycinin. Biochim Biophys Acta 412: 214–228.
Gatehouse JA, Croy RRD, Morton H, Tyler M, Boulter D (1981) Characterization and subunit structure of the vicilin storage proteins of pea (Pisum sativum L.). Eur J Biochem 118: 627–633.
Mori T, Utsumi S (1979) Purification and properties of storage proteins of broad bean. Agric Biol Chem 43: 577–583.
Marcone MF, Beniac DR, Harauz G, Yada RY (1994) Quaternary structure and model for the oligomeric seed globulin from Amaranthus hypochondricus K343. J Agric Food Chem 42: 2675–2678.
Marcone MF, Kakuda K, Yada RY (1998b) Salt-soluble seed globulins of various dicotyledonous and monocotyledonous plants. II. Structural characterization. Food Chem 63: 265–274.
German B, Damodaran S, Kinsella JE (1982) Thermal dissociation and association behaviour of soy proteins. J Agric Food Chem 30: 807–811.
Damodaran S (1988) Refolding of thermally unfolded soy proteins during the cooling regime of the gelation process: Effect on gelation. J Agric Food Chem 36: 262–269.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Marcone, M.R., Kakuda, Y., Jahaniaval, F. et al. Characterization of the proteins of Pili nut (Canarium ovatum, Engl.). Plant Foods Hum Nutr 57, 107–120 (2002). https://doi.org/10.1023/A:1015266423254
Issue Date:
DOI: https://doi.org/10.1023/A:1015266423254