Abstract
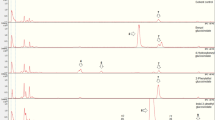
Interactions between insects and glucosinolate-containing plant species have been investigated for a long time. Although the glucosinolate–myrosinase system is believed to act as a defense mechanism against generalist herbivores and fungi, several specialist insects use these secondary metabolites for host plant finding and acceptance and can handle them physiologically. However, sequestration of glucosinolates in specialist herbivores has been less well studied. Larvae of the turnip sawfly Athalia rosae feed on several glucosinolate-containing plant species. When larvae are disturbed by antagonists, they release one or more small droplets of hemolymph from their integument. This “reflex bleeding” is used as a defense mechanism. Specific glucosinolate analysis, by conversion to desulfoglucosinolates and analysis of these by high-performance liquid chromatography coupled to diode array UV spectroscopy and mass spectrometry, revealed that larvae incorporate and concentrate the plant's characteristic glucosinolates from their hosts. Extracts of larvae that were reared on Sinapis alba contained sinalbin, even when the larvae were first starved for 22 hr and, thus, had empty guts. Hemolymph was analyzed from larvae that were reared on either S. alba, Brassica nigra, or Barbarea stricta. Leaves were analyzed from the same plants the larvae had fed on. Sinalbin (from S. alba), sinigrin (B. nigra), or glucobarbarin and glucobrassicin (B. stricta) were present in leaves in concentrations less than 1 μmol/g fresh weight, while the same glucosinolates could be detected in the larvae's hemolymph in concentrations between 10 and 31 μmol/g fresh weight, except that glucobrassicin was present only as a trace. In larval feces, only trace amounts of glucosinolates (sinalbin and sinigrin) could be detected. The glucosinolates were likewise found in freshly emerged adults, showing that the sequestered phytochemicals were transferred through the pupal stage.
Similar content being viewed by others
REFERENCES
ABE, F., YAMAUCHI, T., HONDA, K., OMURA, H., and HAYASHI, N. 2001. Sequestration of phenanthroindolizidine alkaloids by an Asclepiadaceae-feeding danaid butterfly, Ideopsis similis. Phytochemistry 56:697–701.
AGERBIRK, N., OLSEN, C. E., and NIELSEN, J. K. 2001a. Seasonal variation in leaf glucosinolates and insect resistance in two types of Barbarea vulgaris ssp. arcuata. Phytochemistry 58:91–100.
AGERBIRK, N., PETERSEN, B. L., OLSEN, C. E., HALKIER, B. A., and NIELSEN, J. K. 2001b. 1,4-Dimethoxyglucobrassicin in Barbarea and 4-hydroxyglucobrassicin in Arabidopsis and Brassica. J. Agric. Food Chem. 49:1502–1507.
AMANO, T., NISHIDA, R., KUWAHARA, Y., and FUKAMI, H. 1999. Pharmacophagous acquisition of clerodendrins by the turnip sawfly (Athalia rosae ruficornis) and their role in the mating behavior. Chemoecology 9:145–150.
APLIN, R. T., D'ARCY WARD, R., and ROTHSCHILD, M. 1975. Examination of the large white butterfly and small white butterflies (Pieris spp.) for the presence of mustard oils and mustard oil glycosides. J. Entomol. A 50:73–78.
BLUM, M. S. 1981. Chemical Defenses of Arthropods. Academic Press, New York.
BOWERS, M. D. 1992. The evolution of unpalatability and the cost of chemical defense in insects, pp. 216–244, in B. D. Roitberg and M. B. Isman (eds.). Insect Chemical Ecology. An Evolutionary Approach. Chapman and Hall, New York.
BOWERS, M. D., BOOCKVAR, K., and COLLINGE, S. K. 1993. Iridoid glycosides of Chelone glabra (Scrophulariaceae) and their sequestration by larvae of the sawfly, Tenthredo grandis (Tenthredinidae). J. Chem. Ecol. 19:815–823.
BRATTSTEN, L. B. 1992. Metabolic defenses against plant allelochemicals, pp. 175–242, in G. A. Rosenthal and M. R. Berenbaum (eds.). Herbivores: Their Interactions with Secondary Plant Metabolites, Vol. II: Evolutionary and Ecological Processes. Academic Press, New York.
BROWER, L. P. and FINK, L. S. 1985. A natural toxic defense system: Cardenolides in butterflies versus birds. Ann. NY Acad. Sci. 443:171–186.
BUCHNER, R. 1987. Approach to determination of HPLC response factors for glucosinolates, pp. 50–58, in J.-P. Wathelet (ed.). Glucosinolates in Rapeseeds: Analytical Aspects. Martinus Nijhoff Publishers, Dordrecht, The Netherlands.
CARROLL, M., HANLON, A., HANLON, T., ZANGERL, A. R., and BERENBAUM, M. R. 1997. Behavioral effects of carotenoid sequestration by the parsnip webworm, Depressaria pastinacella. J. Chem. Ecol. 23:2707–2719.
CHEW, F. S. 1988a. Searching for defensive chemistry in the Cruciferae, or, do glucosinolates always control interactions of Cruciferae with their potential herbivores and symbionts? No!, pp. 81–112, in K. C. Spencer (ed.). Chemical Mediation of Coevolution. Academic Press, Toronto, Canada.
CHEW, F. S. 1988b. Biological effects of glucosinolates, pp. 155–181, in H. G. Cutler (ed.). Biologically Active Natural Producs-Potential Use in Agriculture. American Chemical Society Symposium, Washington, D.C.
CODELLA, S. G., and RAFFA, K. F. 1993. Defense strategies of folivorous sawflies, pp. 261–294, in M. R. Wagner and K. F. Raffa (eds.). Sawfly Life History. Adaptations toWoody Plants. Academic Press, San Diego, California.
DOBLER, S., DALOZE, D., and PASTEELS, J.M. 1998. Sequestration of plant compounds in a leaf beetle's defensive secretion: Cardenolides in Chrysochus. Chemoecology 8:111–118.
DOBLER, S., HABERER,W., WITTE, L., and HARTMANN, T. 2000. Selective sequestration of pyrrolizidine alkaloids from diverse host plants by Longitarsus flea beetles. J. Chem. Ecol. 26:1281–1298.
DUFFEY, S. S. 1980. Sequestration of plant natural products by insects. Annu. Rev. Entomol. 25:447–477.
EISNER, T., JOHANESSEE, J. S., CARREL, J., HENDRY, L. B., and MEINWALD, J. 1974. Defensive use by an insect of a plant resin. Science 184:996–999.
FAHEY, J. W., ZALCMANN, A. T., and TALALAY, P. 2001. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 56:5–51.
HALKIER, B. A. 1999. Glucosinolates, pp. 193–223, in R. Ikan (ed.). Naturally Occuring Glycosides. John Wiley & Sons Ltd., London.
HEADS, P. A. 1986. Bracken, ants and extrafloral nectaries. IV. Dowood ants (Formica lugubris) protect the plant against insect herbivores? J. Anim. Ecol. 55:795–809.
HEADS, P. A. and LAWTON, J. H. 1985. Bracken, ants and extrafloral nectaries. III. Howinsect herbivores avoid ant predation. Ecol. Entomol. 10:29–42.
HODGSON, E. and ROSE, R. 1991. Insect Cytochrome P 450, pp. 75–91, in E. Arinc, J. B. Schenkman, and E. Hodgson (eds.). Molecular Aspects of Monooxygenases and Bioactivation of Toxic Compounds. Plenum Press, New York.
ISMAN, M. B. 1992. A physiological perspective, pp. 156–176, in B. D. Roitberg and M. B. Isman (eds.). Insect Chemical Ecology. An Evolutionary Approach. Chapman and Hall, New York.
KUNZE, A., AREGULLIN, M., RODRIGUEZ, E., and PROKSCH, P. 1996. Fate of the chromene encecalin in the interactions of Encelia farinosa and its specialized herbivore Trirhabda geminata. J. Chem. Ecol. 22:491–498.
LI, Q., EIGENBRODE, S. D., STRINGHAM, G. R., and THIAGARAJAH, M. R. 2000. Feeding and growth of Plutella xylostella and Spodoptera eridania on Brassica juncea with varying glucosinolate concentrations and myrosinase activities. J. Chem. Ecol. 26:2401–2419.
LISTON, A. 1995. Compendium of European sawflies. Chalastos Forestry, Gottfrieding, Germany.
LOUDA, S. and MOLE, S. 1991. Glucosinolates: Chemistry and ecology, pp. 123–164, in G. A. Rosenthal and M. R. Berenbaum (eds.): Herbivores and Their Interactions with Secondary Plant Metabolites 2E (1): Chemical Participants. Academic Press, New York.
MANICI, L. M., LAZZERI, L., and PALMIERI, S. 1997. In vitro fungitoxic activity of some glucosinolates and their enzyme-derived products towards plant pathogenic fungi. J. Agric. Food Chem. 45:2768–2773.
MCGREGOR, D. I. 1985. Determination of glucosinolates in Brassica seed. Eucarpia Cruciferae Newsl. 10:132–136.
MITHEN, R. F., DEKKER, M., VERVERK, R., RABOT, S., and JOHNSON, I. T. 2000. The nutritional significance, biosynthesis and bioavailability of glucosinolates in human foods. J. Sci. Food Agric. 80:967–984.
MORROW, P. A., BELLAS, T. E., and EISNER, T. 1976. Eucalyptus oil in the defensive oral discharge of Australian sawfly larvae (Hymenoptera: Pergidae). Oecologia 24:193–206.
MURRAY, C. L., QUAGLIA, M., ARNASON, J. T., and MORRIS, C. E. 1994. A putative nicotine pump at the metabolic blood-brain barrier of the tobacco hornworm. J. Neurobiol. 25:23–34.
NIELSEN, J. K. 1988. Crucifer-feeding Chrysomelidae: Mechanisms of host plant finding and acceptance, pp. 25–40, in P. Jolivet, E. Petitpierre, and T. H. Hsiao (eds.): Biology of Chrysomelidae. Kluwer Academic Publishers, Dordrecht, The Netherlands.
NIELSEN, J. K., HANSEN, M. L., AGERBIRK, N., PETERSEN, B. L., and HALKIER, B. A. 2001. Responses of the flea beetles Phyllotreta nemorum and P. cruciferae to metabolically engineered Arabidopsis thaliana with an altered glucosinolate profile. Chemoecology 11:75–83.
NISHIDA, R. and FUKAMI, H. 1990. Sequestration of distasteful compounds by some pharmacophagous insects. J. Chem. Ecol. 16:151–164.
OHARA, Y., NAGASAKI, K., and OHSAKI, N. 1993. Warning coloration in sawfly Athalia rosae larva and concealing coloration in butterfly Pieris rapae larva on similar plants evolved through individual selection. Res. Popul. Ecol. 19:223–230.
POTTER, M. J., VANSTONE, V. A., DAVIES, K. A., and RATHJEN, A. J. 2000. Breeding to increase the concentration of 2-phenylethyl glucosinolate in the roots of Brassica napus. J. Chem. Ecol. 26:1811–1820.
PROP, N. 1960. Protection against birds and parasites in some species of tenthredinid larvae. Arch. Neerl. Zool. 13:380–447.
RASK, L., ANDRéASSON, E., EKBOM, B., ERIKSSON, S., PONTOPPIDAN, B., and MEIJER, J. 2000. Myrosinase: Gene family evolution and herbivore defense in Brassicaceae. Plant Mol. Biol. 42:93–113.
RENWICK, J. A. A., RADKE, C. D., SACHDEV-GUPTA, K., and STäDLER, E. 1992. Leaf surface chemicals stimulating oviposition by Pieris rapae (Lepidoptera: Pieridae) on cabbage. Chemoecology 3:33–38.
RODMAN, J. E., SOLTIS, P. S., SOLTIS, D. E., SYTSMA, K. J., and KAROL, K. G. 1998. Parallel evolution of glucosinolate biosynthesis inferred from congruent nuclear and plastid gene phylogenies. Am. J. Bot. 85:997–1006.
ROTHSCHILD, M. and EDGAR, J. A. 1978. Pyrrolizidine alkaloids from Senecio vulgaris sequestered and stored by Danaus plexippus. J. Zool. 186:347–349.
SANG, J. P., MINCHINTON, I. R., JOHNSTONE, P. K., and TRUSCOTT, R. J. W. 1984. Glucosinolate profiles in the seed, root and leaf tissue of cabbage, mustard, rapeseed, radish and swede. Can. J. Plant Sci. 64:77–93.
SCHAFFNER, U. and BOEVé, J.-L. 1996. Sequestration of plant alkaloids by the sawfly Rhadinoceraea nodicornis: Ecological relevance for different life stages and occurence among related species. Entomol. Exp. Appl. 80:283–285.
SCHAFFNER, U., BOEVé, J.-L., GFELLER, H., and SCHLUNEGGER, U. P. 1994. Sequestration of Veratrum alkaloids by specialist Rhadinoceraea nodicornis Konow (Hymenoptera, Tenthredinidae) and its ecoethological implications. J. Chem. Ecol. 20:3233–3250.
SCHITTKO, U., BURGHARDT, F., FIEDLER, K., WRAY, V., and PROKSCH, P. 1999. Sequestration and distribution of flavonoids in the common blue butterfly Polyommatus icarus reared on Trifolium repens. Phytochemistry 51:609–614.
SCUDDER, G. G. E., MOORE, L. V., and ISMAN, M. B. 1986. Sequestration of cardenolides in Oncopeltus fasciatus: Morphological and physiological adaptations. J. Chem. Ecol. 12:1171–1187.
SEVASTOPULO, D. G. 1958. Defensive action of a sawfly larva (Hym., Symphyta). Entomol. Month. Mag. 94:275.
TRIGO, J. R. 2000. The chemistry of antipredator defense by secondary compounds in neotropical Lepidoptera: Facts, perspectives and caveats. J. Braz. Chem. Soc. 11:551–561.
WITTHOHN, K. and NAUMANN, C. M. 1987. Cyanogenesis—a general phenomenon in the Lepidoptera? J. Chem. Ecol. 13:1789–1809.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Müller, C., Agerbirk, N., Olsen, C.E. et al. Sequestration of Host Plant Glucosinolates in the Defensive Hemolymph of the Sawfly Athalia rosae. J Chem Ecol 27, 2505–2516 (2001). https://doi.org/10.1023/A:1013631616141
Issue Date:
DOI: https://doi.org/10.1023/A:1013631616141