Abstract
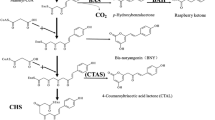
Three polyketide synthase genes (PKS1, PKS2, PKS3) from cell suspension cultures of raspberry (Rubus idaeus L. cv. Royalty) were characterized. They showed high similarity in both their nucleotide and deduced amino acid sequences. All three proteins contain the amino acid residues identified in previous work as essential for chalcone synthase (CHS) function. Enzyme activities were investigated after heterologous expression inEscherichia coli. RiPKS1 is a typical naringenin CHS that synthesizes the chalcone as the main reaction product, and p-coumaryltriacetic acid lactone (CTAL) as a minor by-product. RiPKS3 differed from RiPKS1 in four positions (K49R, M64R, P120L, V188A), and the products in vitro were predominantly CTAL and low levels of chalcone. RiPKS2 had the same four differences from RiPKS1 as RiPKS3, but in addition two further exchanges (R259H, F344L), and the protein had no detectable enzyme activity. Experiments with RiPKS1 containing either 259H or 344L showed that each of the exchanges was sufficient to completely eliminate enzyme activity. These experiments identify amino acid residues in CHS which are important for folding of the tetraketide intermediate to the chalcone (PKS3) and which are in general essential for CHS activity (PKS2). The possible functions of these residues are discussed.
Similar content being viewed by others
References
Aatsinki, J.T., Lakkakorpi, J.T., Pietila, E.M. and Rajaniemi, H.J. 1994. A coupled one-step reverse transcription PCR procedure for generation of full-length open reading frames. Biotechniques 16: 282–286.
Akiyama, T., Shibuya, M., Liu, H.M. and Ebizuka, Y. 1999. p—Coumaroyltriacetic acid synthase, a new homologue of chalcone synthase, from Hydrangea macrophylla var. thunbergii. Eur. J. Biochem. 263: 834–839.
Altschul, S.F., Gish, W., Miller, W., Myers, E.W. and Lipman, D.J. 1990. Basic Local Alignment Search Tool. J. Mol. Biol. 215: 403–410.
Barritt, B.H. and Torre, L.C. 1975. Fruit anthocyanin pigments of red raspberry cultivars. J. Am. Soc. Hort. Sci. 100: 98–99.
Beerhues, L. 1996. Benzophenone synthase from cultured cells of Centaurium erythraea. FEBS Lett. 383: 264–266.
Borejsza-Wysocki, W. and Hrazdina, G. 1994. Biosynthesis of p-hydroxyphenylbutan-2-one in raspberry fruits and tissue cultures. Phytochemistry 35: 623–628.
Borejsza-Wysocki, W. and Hrazdina, G. 1996. Aromatic polyketide synthases in Rubus: purification, characterization and antibody development to benzalacetone synthase from raspberry fruits. Plant Physiol. 110: 791–799.
Bradford, M.M. 1976. A rapid and sensitive method for quantitation of microgram quantities of protein using the principle of protein-dye binding. Anal. Biochem. 72: 248–254.
Christensen, A.B., Gregersen, P.L., Schröder, J. and Collinge, D.B. 1998. A chalcone synthase with an unusual substrate preference is expressed in barley leaves in response to UV light and pathogen attack. Plant Mol. Biol. 37: 849–857.
Ferrer, J.L., Jez, J.M., Bowman, M.E., Dixon, R.A. and Noel, J.P. 1999. Structure of chalcone synthase and the molecular basis of plant polyketide biosynthesis. Nature Struct. Biol. 6: 775–784.
Gehlert, R. and Kindl, H. 1991. Induced formation of dihydrophenanthrenes and bibenzyl synthase upon destruction of orchid mycorrhiza. Phytochemistry 30: 457–460.
Helariutta, Y., Elomaa, P., Kotilainen, M., Griesbach, R.J., Schröder, J. and Teeri, T.H. 1995. Chalcone synthase-like genes active during corolla development are differentially expressed and encode enzymes with different catalytic properties in Gerbera hybrida (Asteraceae). Plant Mol. Biol. 28: 47–60.
Herderich, M., Beckert, C., and Veit, M. 1997. Establishing styrylpyrone synthase activity in cell free extracts obtained from gametophytes of Equisetum arvense L. by high performance liquid chromatography-tandem mass spectrometry. Phytochem. Anal. 8: 194–197.
Hrazdina, G., Lifson, E. and Weeden, N.F. 1986. Isolation and characterization of buckwheat (Fagopyrum esculentum M.) chalcone synthase and its polyclonal antibodies. Arch. Biochem. Biophys. 247: 414–419.
Ishikura, N. and Sugahara, K. 1979. A survey of anthocyanin in fruits of some angiosperms. Bot. Mag. Tokyo. 92: 157–159.
Jez, J.M., Ferrer, J.L., Bowman, M.E., Dixon, R.A. and Noel, J.P. 2000. Dissection of malonyl-coenzyme A decarboxylation from polyketide formation in the reaction mechanism of a plant polyketide synthase. Biochemistry 39: 890–902.
Junghanns, K.T., Kneusel, R.E., Baumert, A., Maier, W., Gröger, D. and Matern, U. 1995. Molecular cloning and heterologous expression of acridone synthase from elicited Ruta graveolens L. cell suspension cultures. Plant Mol. Biol. 27: 681–692.
Kohalmi, S.E. and Kunz, B.A. 1988. Role of neighboring bases and assessment of strand specificity in ethylmethanesulphonate and N-methyl-N'-nitro-N-nitrosoguanidine mutagenesis in the SUP4-o gene of Saccharomyces cerevisiae. J. Mol. Biol. 204: 561–568.
Martin, C.R. 1993. Structure, function, and regulation of the chalcone synthase. Int. Rev. Cytol. 147: 233–284.
Möller, E.M., Bahnweg, G., Sandermann, H. and Geiger, H.H. 1992. A simple and efficient protocol for isolation of high molecular weight DNA from filamentous fungi, fruit bodies and infected plant tissues. Nucl. Acid Res. 20: 6115–6116.
Ochman, H., Gerber, A.S. and Hartl, D.L. 1990. Genetic application of an inverse polymerase chain reaction. Genetics 120: 621–623.
Preisig-Müller, R., Gnau, P. and Kindl, H. 1995. The inducible 9,10-dihydrophenanthrene pathway: characterization and expression of bibenzyl synthase and S-adenosylhomocysteine hydrolase. Arch. Biochem. Biophys. 317: 201–207.
Raiber, S., Schröder, G. and Schröder, J. 1995. Molecular and enzymatic characterization of two stilbene synthases from Eastern white pine (Pinus strobus): a single Arg/His difference determines the activity and the pH dependence of the enzymes. FEBS Lett. 361: 299–302.
Reinecke, T. and Kindl, H. 1994. Characterization of bibenzyl synthase catalysing the biosynthesis of phytoalexins of orchids. Phytochemistry 35: 63–66.
Rommel, A. and Wrolstad, R.E. 1993. Composition of flavonols in red raspberry juice as influenced by cultivar, processing, and environmental factors. J. Agric. Food Chem. 41: 1941–1950.
Sambrook, J., Fritsch, E.F. and Maniatis, T. 1989. Molecular Cloning: A Laboratory Manual, 2nd ed. Cold Spring Harbor Laboratory Press, Plainview, NY.
Sarkar, G., Turner, R.T. and Bolander, M.A. 1993. Restriction-site PCR: a direct method of unknown sequence retrieval adjacent to a known locus by using universal primers. PCR Meth. Appl. 2: 318–322.
Schinz, H. and Seidel, C.F. 1957. Untersuchungen über Aro-mastoffe. 1. Über das Himbeeraroma. Helv. Chim. Acta 40: 1839–1859.
Schröder, J. 1997. A family of plant-specific polyketide synthases: facts and predictions. Trends Plant Sci. 2: 373–378.
Schröder, J., Raiber, S., Berger, T., Schmidt, A., Schmidt, J., Soares-Sello, A.M., Bardshiri, E., Strack, D., Simpson, T.J., Veit, M. and Schröder, G. 1998. Plant polyketide synthases: a chalcone synthase-type enzyme which performs a condensation reaction with methylmalonyl-CoA in the biosynthesis of C-methylated chalcones. Biochemistry 37: 8417–8425.
Shirley, B.W., Kubasek, W.L., Storz, G., Bruggemann, E., Koorneef, M., Ausubel, F.M. and Goodman, H.M. 1995. Analysis of Arabidopsis mutants deficient in flavonoid biosynthesis. Plant J. 8: 659–671.
Sütfeld, R. and Wiermann, R. 1980. Chalcone synthesis with enzyme extracts from tulip anther tapetum using a biphasic enzyme assay. Arch. Biochem. Biophys. 201: 64–72.
Torre, L.C. and Barritt, B.H. 1977. Quantitative evaluation of Rubus fruit anthocyanin pigments. J. Food. Sci. 42: 488–490.
Yamaguchi, T., Kurosaki, F., Suh, D.Y., Sankawa, U., Nishioka, M., Akiyama, T., Shibuya, M. and Ebizuka, Y. 1999. Cross-reaction of chalcone synthase and stilbene synthase over-expressed in Escherichia coli. FEBS Lett. 460: 457–461.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zheng, D., Schröder, G., Schröder, J. et al. Molecular and biochemical characterization of three aromatic polyketide synthase genes from Rubus idaeus. Plant Mol Biol 46, 1–15 (2001). https://doi.org/10.1023/A:1010642517738
Issue Date:
DOI: https://doi.org/10.1023/A:1010642517738