Abstract
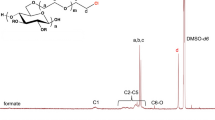
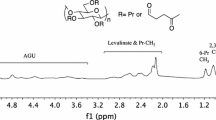
Estimation of the substitution distribution for cyanoethyl cellulose was carried out by 1H and 13C NMR spectroscopic analyses after the additional acetylation. Based on the complemental function of cyanoethyl and acetyl substituents, the degree of substitution (DS) of cyanoethyl groups could be calculated from the ratios of the 1H integrated intensities in acetyl methyl (δ 1.8–2.1) and cyanoethyl methylene (δ 2.6–2.9) protons, and also from the corresponding ratios for acetyl methyl carbon signals (δ 19.7–21.3) and cyanoethyl methylene carbon signals (δ 17.5–19.0). Good agreement was obtained between the DS values obtained from the NMR spectroscopic analyses and those determined by the conventional nitrogen content method, indicating the validity of the NMR method used. In addition, the NMR method was found to be effective in determining the positional substituent distribution by the quantitative analysis of the three cyan carbon signals (δ 118–120).
Similar content being viewed by others
REFERENCES
Goodlett V. W., Dougherty J. T. and Patton H. W. (1971) Characterization of cellulose acetates by nuclear magnetic resonance. J. Polym. Sci. A-1 9, 155-161.
Isogai A., Ishizu A. and Nakano J. (1984) Distribution of substituents in cellulose ethers prepared in aqueous and non-aqueous systems. Sen-i Gakkaishi 40, 504-511.
Iwata T., Azuma J., Okamura K. Muramoto M and Chun B. (1992) Preparation and NMR assignments of cellulose mixed esters regioselectively substituted by acetyl and propanoyl groups. Carbohydr. Res. 224, 277-283.
Kamide K. and Okajima K. (1981) Determination of distribution of O-acetyl group in trihydric alcohol units of cellulose acetate by carbon-13 nuclear magnetic resonance analysis. Polym. J. 13, 127-133.
Keely C. M., Zhang X. and McBrierty M. J. (1995) Hydration and plasticization effects in cellulose acetate: a solid-state NMR study. J. Mol. Struc. 355, 33-46.
Kowsaka K., Okajima K. and Kamide K. (1986) Further study on the distribution of substituent group in cellulose acetate by 13C{1H} NMR analysis: Assignment of carbonyl carbon peaks. Polym. J. 18, 843-849.
Miyamoto T., Sato Y., Shibata T. and Inagaki H. (1984) 13C nuclear magnetic resonance studies of cellulose acetate. J. Polym. Sci: Polym. Chem. Ed. 22, 2363-2370.
Nakayama E., Kanei M., Kohara N. and Toyoda H. (1992) Cyanoethylation of synthetic glucan and dielectric properties of products. Sen-i Gakkaishi 48, 461-466.
Nakayama E., Kohara N. and Toyoda H. (1995) Dielectric properties of cyanoethyl amylopectins. Sen-i Gakkaishi 51, 200-206.
Ohno Y., Nakayama E. and Kohara N. (1987) Studies of the cyanoethyl cellulose (part 2) Synthesis of cyanoethyl acetyl cellulose. Bull. Showa Women's Univ. 576, 118-109.
Reuben J. (1986) Analysis of the 13C NMR spectra of hydrolyzed and methanolyzed Omethylcelluloses: Monomer compositions and models for their description. Carbohydr. Res. 157, 201-213.
Shuto Y., Murayama M., Azuma J. and Okamura K. (1988) NMR assignments of propionyl groups in cellulose tripropionate. Bull. Inst. Chem. Res., Kyoto Univ. 66, 128-135.
Takahashi S., Fujimoto T., Barua B.M. and Miyamoto T. (1986) 13C NMR spectral studies on the distribution of substituents in some cellulose derivatives. J. Polym. Sci: Polym. Chem. Ed. 24, 2981-2993.
Tezuka Y., Imai K., Oshima M. and Chiba T. (1987) Determination of substituent distribution in cellulose ethers by means of a 13C NMR study on their acetylated derivatives. 1. Methylcellulose. Macromolecules 20, 2413-2418.
Tezuka Y., Tsuchiya Y. and Shiomi T. (1996) 13C NMR determination of substituent distribution in carboxymethylcellulose by use of its peresterified derivatives. Carbohydr. Res. 291, 99-108.
Verraest D. L., Peters J. A., Kuzee H. C., Raaijmakers H. W. C. and Bekkum H. (1997) Distribution of substituents in O-carboxymethyl and O-cyanoethyl ethers of inulin. Carbohydr. Res. 302, 203-212.
Wu T. K. (1980) Carbon-13 and proton nuclear magnetic resonance studies of cellulose nitrates. Macromolecules 13, 74-79.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nakayama, E., Azuma, JI. Substituent Distribution of Cyanoethyl Cellulose. Cellulose 5, 175–185 (1998). https://doi.org/10.1023/A:1009276916877
Issue Date:
DOI: https://doi.org/10.1023/A:1009276916877