Abstract
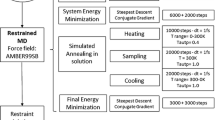
The inclusion of explicit solvent water in molecular dynamics refinement of NMR structures ought to provide the most physically meaningful accounting for the effects of solvent on structure, but is computationally expensive. In order to evaluate the validity of commonly used vacuum refinements and of recently developed continuum solvent model methods, we have used three different methods to refine a set of NMR solution structures of a medium sized protein, Escherichia coliglutaredoxin 2, from starting structures calculated using the program DYANA. The three different refinement protocols used molecular dynamics simulated annealing with the program AMBER in vacuum (VAC), including a generalized Born (GB) solvent model, and a full calculation including explicit solvent water (WAT). The structures obtained using the three methods of refinements were very similar, a reflection of their generally well-determined nature. However, the structures refined with the generalized Born model were more similar to those from explicit water refinement than those refined in vacuum. Significant improvement was seen in the percentage of backbone dihedral angles in the most favored regions of φ,ψ space and in hydrogen bond pattern for structures refined with the GB and WAT models, compared with the structures refined in vacuum. The explicit water calculation took an average of 200 h of CPU time per structure on an SGI cluster, compared to 15–90 h for the GB calculation (depending on the parameters used) and 2 h for the vacuum calculation. The generalized Born solvent model proved to be an excellent compromise between the vacuum and explicit water refinements, giving results comparable to those of the explicit water calculation. Some improvement for φ and ψ angle distribution and hydrogen bond pattern can also be achieved by energy minimizing the vacuum structures with the GB model, which takes a much shorter time than MD simulations with the GB model.
Similar content being viewed by others
References
Bashford, D. and Case, D.A. (2000) Annu. Rev. Phys. Chem., 51, 129–152.
Calimet, N., Schaefer, M. and Simonson, T. (2001) Proteins, 45, 144–158.
Case, D.A., Pearlman, D.A., Caldwell, J.W., Cheatham, III, T.E., Ross, W.S., Simmerling, C.L., Darden, T.A., Merz, K.M., Stanton, R.V., Cheng, A.L., Vincent, J.J., Crowley, M., Tsui, V., Radmer, R.J., Duan, Y., Pitera, J., Massova, I., Seibel, G.L., Singh, U.C., Weiner, P.K. and Kollman, P.A. (1999). AMBER 6, University of California, San Francisco, CA.
Cheatham, III, T.E. and Kollman, P.A. (1996) J. Mol. Biol., 259, 434–444.
Cornell, W., Abseher, R., Nilges M. and Case, D.A. (2001) J. Mol. Graph. Model., 19, 136–145.
Cramer, C.J. and Truhlar, D.G. (1999) Chem. Rev., 99, 2161–2200.
Dauter, Z., Lamzin, V.S. and Wilson, K.S. (1997) Curr. Opin. Struct. Biol., 7, 681–688.
Dominy, B.N. and Brooks, III, C.L. (1999) J. Phys. Chem., 103, 3765–3773.
Güntert, P., Mumenthaler, C. and Wüthrich, K. (1997) J. Mol. Biol., 273, 283–298.
Koradi, R., Billeter, M. and Wüthrich, K. (1996) J. Mol. Graphics, 14, 51–55.
Laskowski, R.A., Rullmann, J.A.C., MacArthur, M.W., Kaptein, R. and Thornton, J.M. (1996) J. Biomol. NMR, 8, 477–486.
Nicholls, P. (2000) Cell Mol. Life Sci., 57, 987–992.
Onufriev, A., Bashford, D. and Case, D.A. (2000) J. Phys. Chem., B104, 3712–3720.
Otting, G. and Wüthrich, K. (1989) J. Am. Chem. Soc., 111, 1871–1875.
Qiu, D., Shenkin, P.S., Hollinger, F.P. and Still, W.C. (1997) J. Phys. Chem. A, 101, 3005–3014.
Rapp, C.S. and Friesner, R.A. (1999) Proteins, 35, 173–183.
Rodriguez, R., Chinea, G., Lopez, N., Pons, T. and Vriend, G. (1998) Bioinformatics. 14, 523–528.
Schaefer, M., Bartels, C. and Karplus, M. (1998) J. Mol. Biol., 284, 835–848.
Still, W.C., Tempczyk, A., Hawley, R.C. and Hendrickson, T. (1990) J. Am. Chem. Soc., 112, 6127–6129.
Tsui, V. and Case, D.A. (2000) J. Am. Chem. Soc., 122, 2489–2498.
Tsui, V. and Case, D.A. (2001) Biopolymers (Nucl. Acid Sci.), 56, 275–291.
Williams, D.J. and Hall, K.B. (1999) Biophys. J., 76, 3192–3205.
Xia, B., Vlamis-Gardikas, A., Holmgren, A., Wright, P.E. and Dyson, H.J. (2001) J. Mol. Biol., 310, 907–918.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xia, B., Tsui, V., Case, D.A. et al. Comparison of protein solution structures refined by molecular dynamics simulation in vacuum, with a generalized Born model, and with explicit water. J Biomol NMR 22, 317–331 (2002). https://doi.org/10.1023/A:1014929925008
Issue Date:
DOI: https://doi.org/10.1023/A:1014929925008