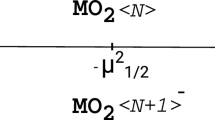
Abstract
The spatial distribution of potassium on an Rh(110) surface during the catalytic O2+H2 reaction is investigated employing photoelectron emission microscopy (PEEM) and scanning photoelectron microscopy (SPEM) as spatially resolving in situ methods. Depending on the reaction conditions, potassium condenses reversibly into macroscopic islands where it is coadsorbed with oxygen. Mass transport of potassium with the reaction fronts is observed. Differences in the mobility and in the bonding strength of potassium on the “reduced” and on the oxygen-covered surface areas are considered to be the key factors for the formation of the stationary concentration patterns.
Similar content being viewed by others
References
W.D. Mross, Catalysis Rev.-Sci. Eng. 25 (1983) 591.
Physics and Chemistry of Alkali Metal Adsorption, eds. H.P. Bonzel, A.M. Bradshaw and G. Ertl, in: Material Science Monographs 67 (Elsevier, 1989).
Coadsorption, promoters and poisons, in: The Chemical Physics of Solid Surfaces and Heterogeneous Catalysis 6, ser. eds. D.A. King and D.P. Woodruff (Elsevier, 1993).
M. Kiskinova, Poisoning and Promotion in Catalysis Based on Surface Science Concepts, in: Studies in Surface Science and Catalysis 70, ser. eds. B. Delmon and J.T. Yates (Elsevier, 1992).
M. Kiskinova, E. Di Fabrizio, M. Gentili and M. Marsi, Surf. Rev. Lett. 6 (1999) 265.
G. Comelli et al., Chem. Phys. Lett. 261 (1996) 253; Surf. Sci. Rep. 32 (1998) 165 and references therein.
M. Ehsasi and K. Christmann, Surf. Sci. 194 (1988) 172.
P.R. Norton, in: The Chemical Physics of Solid Surfaces and Heterogeneous Catalysis 4 (Elsevier, 1982), p. 27.
F. Mertens and R. Imbihl, Chem. Phys. Lett. 242 (1995) 221.
A. Makeev and R. Imbihl, J. Chem. Phys. 113 (2000) 3854.
The K coverages were calibrated against the K2p intensity of the Rh(110)-(1 × 3)-K low coverage phase for which the K coverage had been determined with a quartz microbalance to θ K = 0.08. The oxygen coverage of 0.7 ML is estimated based on LEED observations of a mixture of c(2 × 6) and c(2 × 8) oxygen overlayers (see ref. 6).
G. Pirug, C. Ritke and H.P. Bonzel, Surf. Sci. 257 (1991) 50.
H.P. Bonzel, G. Broden and H.J. Krebs, Appl. Surf. Sci. 16 (1983) 373; M. Kiskinova, G. Pirug and H.P. Bonzel, Surf. Sci. 133 (1983) 321.
N. Al-Sarraf, J.T. Stuckless and D.A. King, Nature 360 (1992) 243.
K. Jacobi, H. Shi, M. Gruyters and G. Ertl, Phys. Rev. B 49 (1994) 5733.
In preparation.
S. Günther, H. Marbach, A. Baraldi, S. Lizzit, M. Kiskinova and R. Imbihl, in preparation.
F. Esch, private communication.
H. Marbach, M. Hinz, S. Günther, R. Imbihl, L. Gregoratti and M. Kiskinova, submitted to Chem. Phys. Lett.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Marbach, H., Günther, S., Luerßen, B. et al. Selforganization of Alkali Metal on a Catalytic Metal Surface. Catalysis Letters 83, 161–164 (2002). https://doi.org/10.1023/A:1021073711705
Issue Date:
DOI: https://doi.org/10.1023/A:1021073711705