Abstract
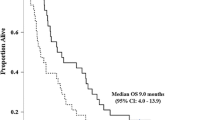
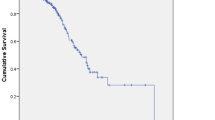
Objectives: To evaluate the efficacy and safety of weekly administration of gemcitabine treatment in chemotherapy-naïve patients with advanced biliary tract and gallbladder cancer. Patients and methods: Gemcitabine at a dose of 800 mg/m2 was administered weekly as a 30-min infusion to patients with previously operated, histologically confirmed, metastatic, or unresectable locally advanced cholangiocarcinoma. Treatment was continued until unacceptable toxicity or disease progression. Results: A total of 30 patients (median age 66 years; range 54–72 years) were included in the study. A median of 14 (range, 4–33) weekly doses was administered. Out of 30 patients evaluable for response, nine partial responses were observed (30.0%), while a further 11 patients demonstrated stable disease (36.7%). The median time to disease progression was 7 months (range, 5–34). Overall response rate was superior in patients with cancer of the gallbladder (ORR=35.7%) compared with those patients with biliary duct cancer (ORR=27.3%). This correlated to a significantly longer time to progression of 6.4 months (95% confidence interval (CI), 5.6–7.1 months) versus 3.6 months (95% CI, 2.9–4.3 months; p=0.03) and a significantly better overall survival of 17.1 months (95% CI, 15.8–18.5 months) versus 11.4 months (95% CI, 10.2–12.6 months, p=0.021). Toxicities were generally mild with only one case of grade 3 neutropenia. There were no cases of febrile neutropenia and no treatment-related deaths. Conclusions: Weekly administration of gemcitabine provides a safe, well-tolerated, and effective treatment for chemotherapy naïve patients with advanced cholangiocarcinoma, particularly with a gallbladder origin.
Similar content being viewed by others
References
Harder J, Blum HE: Chloangiocarcinoma. Schweiz Rundsch Med Prax 91: 1532–1536, 2002
DeGroen PC, Gores GJ, BlaRusso NF et al.: Biliary tract cancers. N Eng J Med 341: 1368–1378, 1999
Falkson G, MacIntyre JM, Moertel CG: Eastern cooperative oncology group experience with chemotherapy for inoperable gallbladder and bile duct cancer. Cancer 54: 965–969, 1984
Taal BG, Audisio RA, Bleiberg H et al.: Phase II trial of mitomycin C (MMC) in advanced gallbladder and biliary tree carcinoma. An EORTC gastrointestinal tract cancer cooperative group study. Ann Oncol 4: 607–609, 1993
Harvey JH, Smith FP, Schein PS: 5-Flurouracil, mitomycin, and doxorubicin (FAM) in carcinoma of the bile tract. J Clin Oncol 2: 1245–1248, 1984
Hejna M, Pruckmayer M, Raderer M: The role of chemotherapy and radiation in the management of biliary cancer. A review of the literature. Eur J Cancer 34: 977–986, 1998
Polyzos A, Nikou G, Giannopoulos A et al.: Chemotherapy of biliary tract cancer with mitomycin-C and 5-fluorouracil biologically modulated by folinic acid. A phase II study. Ann Oncol 7: 644–645, 1996
Burris HA, Moore MJ, Andersen J et al.: Improvements in survival and clinical benefit with gemcitabine as first-line therapy in patients with advanced pancreatic cancer: a randomized trial. J Clin Oncol 15: 2403–2407, 1997
Mezger J, Sauerbruch T, Ko Y et al.: A phase II trial of gemcitabine in gallbladder and biliary tract carcinomas. Onkologie 21: 232–234, 1998
Valencak J, Kornek GV, Raderer M et al.: Gemcitabine for the treatment of advanced biliary tract carcinomas: evaluation of two different dose regimens. Onkologie 22: 498–501, 1999
Penz M, Kornek GV, Raderer M et al.: Phase II of two-weekly gemcitabine in patients with advanced biliary tract cancer. Ann Oncol 12: 183–186, 2001
Gallarado JO, Rubio B, Fodor M et al.: A phase II study of gemcitabine in gallbladder carcinoma. Ann Oncol 12: 1403–1406, 2001
Miller A, Hoogstraten B, Staquet M, Winkler A: Reporting results of treatment. Cancer 47: 207–217, 1981
Anderson JR, Bernstein L, Pike MC: Approximate confidence intervals for probabilities of survival and quantiles in life-table analysis. Biometrics 38: 407–416, 1982
Kaplan EL, Meier P: Non-parametric estimation from incomplete observations. J Am Stat Assoc 53: 458–481, 1958
Kliche KO, Kubsch K, Raida M et al.: Chronomodulated chemotherapy in metastatic gastrointestinal cancer combining 5-FU and sodium folinate with oxaliplatin, irinotecan or gemcitabine: the Jena experience in 79 patients. J Cancer Res Clin Oncol 128: 516–524, 2002
Eckel F, Lersch C, Assmann G, Schulte-Frohlinde E: Toxicity of a 24-hour infusion of gemcitabine in biliary tract and pancreatic cancer: a pilot study. Cancer Invest 20: 180–185, 2002
Kubicka S, Rudolph KL, Tietze MK et al.: Phase II study of systemic gemcitabine chemotherapy for advanced unresectable hepatobiliary carcinomas. Hepatogastroenterology 48: 783–789, 2001
Fossella FV, Lippman SM, Shin DM et al.: Maximum-tolerated dose defined for single agent gemcitabine: A phase I dose-escalation study in chemotherapy-naïve patients with advanced non-small cell lung cancer. J Clin Oncol 15: 310–316, 1997
Ulrich-Pur H, Kornek GV, Raderer M et al.: A phase II trial of biweekly high-dose gemcitabine for patients with metastatic pancreatic adenocarcinoma. Cancer 88: 2505–2511, 2000
Takada T, Kato H, Matsusuhiro T et al.: Comparison of 5-flurouracil, doxorubicin and mitomycin C with 5-flurouracil alone in the treatment of pancreatic-biliary carcinomas. Oncology 51: 396–400, 1994
Patt YZ, Jones DV Jr, Hoque A et al.: Phase II trial of intravenous flurouracil and interferon alfa-2b for biliary tract cancer. J Clin Oncol 14: 2311–2315, 1996
Ellis PA, Norman A, Hill A et al.: Epirubicin, cisplatin, and infusional 5-flurouracil (5-FU) (ECF) in hepatobiliary tumors. Eur J Cancer 31A: 594–598, 1995
Cucreux M, Rougier P, Fandi A et al.: Effective treatment of advanced biliary tract carcinoma using 5-fluoruracil continuous infusion with cisplatin. Ann Oncol 9: 653–656, 1998
Glimelius B, Hoffman K, Sjoden PO et al.: Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol 7: 593–600, 1996
Bramhall SR, Schulz J, Nemunaitis J et al.: A double-blind placebo-controlled, randomized study comparing gemcitabine and marimastat with gemcitabine and placebo as first line therapy in patients with advanced pancreatic cancer. Br J Cancer 87: 161–167, 2002
Cohen SH, Ho L, Ranganathan S et al.: Phase II and pharmacodynamic study of the farnesyltransferase inhibitor R115777 as initial therapy in patients with metastatic pancreatic adenocarcinoma. J Clin Oncol 21: 1301–1306, 2003
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tsavaris, N., Kosmas, C., Gouveris, P. et al. Weekly Gemcitabine for the Treatment of Biliary Tract and Gallbladder Cancer. Invest New Drugs 22, 193–198 (2004). https://doi.org/10.1023/B:DRUG.0000011797.09549.53
Issue Date:
DOI: https://doi.org/10.1023/B:DRUG.0000011797.09549.53