Abstract
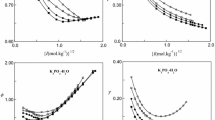
The solubility of oxygen has been measured in a number of electrolytes [(LiCl, KCl, RbCl, CsCl, NaF, NaBr, NaI, NaNO3, KBr, KI, KNO3, CaCl2, SrCl2, BaCl2, Li2SO4, K2SO4, Mn(NO3)3)] as a function of concentration at 25°C. The solubilities, μmol (kg-H2O)−1, have been fitted to a function of the molality m (standard deviation σ < 3μmol-kg−1)
where A and B are adjustable parameters and the activity coefficient of oxygen γ)O2) = [O2]0/[O2]. The limiting salting coefficient, k S = (∂ln γ/∂ m)m=0 = A, was determined for all salts. The salting coefficients for the chlorides and sodium salts showed a near linear correlation with the crystal molar volume V cryst = 2.52 r 3. The salting coefficients determined from the Scaled Particle Theory were in reasonable agreement with the measured values. The activity coefficients of oxygen in the solutions have been interpreted using the Pitzer equation
where \(\lambda _{{\text{O}}_{\text{2}} {\text{i}}} \) is a parameter that accounts for the interaction of O2 with cations (c) and anions (a) with molalities m a and m c, and \(\zeta _{{\text{O}}_{\text{2}} {\text{ca}}}\) accounts for interactions for O2 with the cation and anion pair (c-a). The \(\lambda _{{\text{O}}_{\text{2}} {\text{i}}}\) and \(\zeta _{{\text{O}}_{\text{2}} {\text{ca}}}\) coefficients determined for the most of the ions are in reasonable agreement with the tabulations of Clegg and Brimblecombe. The values of \(\lambda _{{\text{O}}_{\text{2}} {\text{i}}}\) for most of the ions are a linear function of the electrostriction molar volume (Velect = V0 − V cryst).
Similar content being viewed by others
REFERENCES
R. Battino, IUPAC Solubility Data Series: Oxygen and Ozone, Vol. 7 (Pergamon Press, Oxford, 1981).
R. Battino, T. R. Rettitch, and T. Tominaga, J. Phys. Chem. Ref. Data 12, 163(1983).
S. L. Clegg and P. Brimblecombe, Geochim. Cosmochim. Acta 54, 3315(1990).
S. D. Cramer, Ind. Eng. Chem. Process Design Develop. 19, 300(1980).
J. E. Sherwood, F. Stagnitti, M. J. Kokkinn, and W. D. Williams, Limnol. Oceanogr. 36, 235(1991).
F. J. Millero, Marine Chem. 70, 5(2000).
F. J. Millero Physical Chemistry of Natural Waters Wiley (Interscience), New York, 2001).
F. J. Millero, F. Huang, and A. L. Laferriere, Geochim. Cosmochim. Acta 66, 2349(2002).
F. J. Millero, F. Huang, and A. L. Laferriere, Marine Chem. 78, 217(2002).
J. Setschenow, J. Phys. Chem. 4, 117(1899).
M. Randall, and C. F. Failey, Chem. Rev. 4, 211(1927).
F. A. Long and W. F. McDevit, Chem. Rev. 51, 119(1952).
R. A. Pierotti, J. Phys. Chem. 69, 281(1965).
R. A. Pierotti, Chem. Rev. 76, 717(1976).
W. L. Masterton and T. P. Lee, J. Phys. Chem. 74, 1776(1970).
W. L. Masterton, J. Solution Chem. 4, 523(1975).
E. M. Pawllkowski and J. M. Prausnitz, Ind. Eng. Chem. Fund. 22, 86(1983).
K. S. Pitzer, in Activity Coefficients in Electrolyte Solutions, K. S. Pitzer, ed., 2nd edn, Vol. I (CRC Press, Boca Raton, FL, 1991), p. 75.
C. E. Harvie, N. Møller, and J. H. Weare, Geochim. Cosmochim. Acta 48, 723(1984).
A. R. Felmy and J. H. Weare, Geochim. Cosmochim. Acta 50, 2771(1986).
F. J. Millero and D. Pierrot, Aqua. Geochem. 4, 153(1998).
J. M. Simonson, R. N. Roy, J. Connole, L. N. Roy, and D. A. Johnson, J. Solution Chem. 17, 791(1987).
J. M. Simonson, R. N. Roy, D. Mrad, P. Lord, L. N. Roy, and D. A. Johnson, J. Solution Chem. 17, 435(1988).
F. J. Millero and A. Poisson, Deep-Sea Res. 28, 625(1981).
J. H. Carpenter, Limnol. Oceanogr. 10, 141(1965).
P. J. leB. Williams and N.W. Jenkinson, Limnol. Oceanogr. 27, 576(1982).
B. B. Benson and D. KrauseJr., Limnol. Oceanogr. 29, 620(1984).
H. E. Garcia and L. I. Gordon, Limnol. Oceanogr. 37, 1307(1992).
C. V. Krishnan and H. L. Friedman, J. Solution Chem. 3, 727(1974).
N. E. Khomutov and E. I. Kohhik, Russ. J. Phys. Chem. 48, 359(1974).
A. Yasunishi, J. Chem. Eng. Jpn. 10, 89(1977); Kagaku Kogaku Rombun. 4, 185 (1978).
R. Marcus, Chem. Rev. 88, 1480(1988).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Millero, F.J., Huang, F. & Graham, T.B. Solubility of Oxygen in Some 1-1, 2-1, 1-2, and 2-2 Electrolytes as a Function of Concentration at 25°C. Journal of Solution Chemistry 32, 473–487 (2003). https://doi.org/10.1023/A:1025301314462
Issue Date:
DOI: https://doi.org/10.1023/A:1025301314462