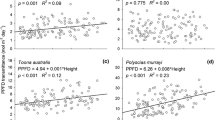
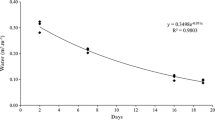
Abstract
It has been hypothesized that plants cannot tolerate combined shade and drought, as a result of morphological trade-offs. However, numerous plant species are reportedly widespread in shaded forest understories that face drought, whether seasonal or occasional. We studied juveniles of six plant species that cope with strong summer drought in the understoreys of mixed Quercus forests in southern Spain: the tall-shrubs Phillyrea latifolia and Viburnum tinus, the perennial herb Rubia peregrina, the small shrub Ruscus aculeatus, and climbers Hedera helix and Smilax aspera. All of these species persist in evergreen shade (c. 3% daylight). Two other species were studied as comparators, Ruscus hypoglossum, less tolerant of drought, and Ceratonia siliqua, less tolerant of shade. Morphological and chemical variables relevant to shade and drought tolerance were measured for juveniles in a range of sizes, and also for the leaves of mature plants. The species converge in features that confer tolerance of shade plus drought by reducing demand for resources. Demand for water is reduced through a moderate to high below-ground mass fraction and low to moderate specific leaf area (respectively 0.22–0.52 and 112–172 cm2 g−1 at 1.00 g total dry mass). Demand for both irradiance and water is reduced through a low to moderate foliar nitrogen concentration and long-lived, physically protected leaves (≥2 yr). The species also converge in features that confer tolerance of either low irradiance or drought through specialized capture of resource, without precluding the other tolerance. These features include deep roots relative to shoot size, moderately higher specific leaf area in shade (1.2–2.0 × that in sun) and higher chlorophyll:nitrogen ratio in shade. Foliar chlorophyll per unit mass was higher in shade, but chlorophyll was not necessarily synthesized in greater amounts; rather, it was higher apparently due to shade effects on structural features linked with specific leaf area. In contrast, N per unit mass was higher in sun leaves independently of specific leaf area. Despite these convergences, the species diverge considerably in their root mass allocation and architecture, leaf saturated water content, density of stomata and guard cell size. No single narrowly defined functional type is needed for tolerance of shade plus drought.
Similar content being viewed by others
References
Abrams M.D. and Mostoller S.A. 1995. Gas exchange, leaf structure and nitrogen in contrasting successional tree species growing in open and understorey sites during a drought. Tree Physiology 15: 361–370.
Aerts R. and Chapin F.S. 2000. The mineral nutrition of wild plants revisited: a re-evaluation of processes and patterns. Advances in Ecological Research 30: 1–67.
Allen S.E. 1989. Chemical Analysis of Ecological Materials. Blackwell, Oxford.
Anderson M.C. 1964. Studies of the woodland light climate. I. The photographic computation of light conditions. Journal of Ecology 52: 27–41.
Antonielli M., Ceccarelli M. and Pocceschi N. 1989. Rubia peregrina L.: a stress resistant weed. Environmental and Experimental Botany 29: 467–476.
Aparicio A. and Silvestre S. 1996. Guía de la flora del Parque Natural Sierra de Grazalema. Junta de Andalucía, Sevilla.
Arber A. 1925. (1961 reprint). Monocotyledons: A Morphological Study. Hafner, New York.
Baldy C., Barbero M. and Madjidieh H. 1987. Extinction du rayonnement et modifications du spectre solaire sous differents couverts du taillis de chêne vert (Quercus ilex L.) de la forêt de la Gardiole de Rians (Var-France). Ecologia Mediterranaea 8: 77–86.
Barbero M., Loisel R. and Quezel P. 1992. Biogeography, ecology and history of Mediterranean Quercus ilex ecosystems. Vegetatio 99: 19–34.
Bell A.D. 1991. (1998 reprint). Plant Form. Oxford University Press, New York.
Björkman O. and Holmgren P. 1963. Adaptability of the photosynthetic apparatus to light intensity in ecotypes from exposed and shaded habitats. Physiologia Plantarum 16: 889–914.
Bongers F. and Popma J. 1988. Is exposure-related variation in leaf characteristics of tropical rain forest adaptive? In: Werger M.J.A., van der Art P.J.N., During H.H. and Verhoeven J.T.A. (eds), Plant Form and Vegetation Structure. SBA Academic Publishing, The Hague, pp. 191–200.
Burslem D.F.R.P. 1996. Differential responses to nutrients, shade and drought among tree seedlings of lowland tropical forest in Singapore. In: Swaine M.D. (ed.), The Ecology of Tropical Forest Tree Seedlings. UNESCO, Paris, and Parthenon, Carnforth, UK, pp. 211–244.
Callaway R.M. 1995. Positive interactions among plants. Botanical Review 61: 306–349.
Canadell J., Djema A., Lopez B., Lloret F., Sabate S., Siscart D. et al. 1999. Structure and dynamics of the root system. In: Roda F., Retana J., Gracia C.A. and Bellot J. (eds), Ecology of Mediterranean Evergreen Forests. Springer-Verlag, Berlin, pp. 47–60.
Canham C.D., Finzi A.C., Pacala S.W. and Burbank D.H. 1994.Causes and consequences of resource heterogeneity in forests: interspecific variation in light transmission by canopy trees.Canadian Journal of Forest Research 24: 337–349.
Canham C.D., Berkowitz A.R., Kelly V.R., Lovett G.M., Ollinger S.V. and Schnurr J. 1996. Biomass allocation and multiple resource limitation in tree seedlings. Canadian Journal of Forest Research 26: 1521–1530.
Caspersen J.P. and Kobe R.K. 2001. Interspecific variation in sapling mortality in relation to growth and soil moisture. Oikos 92: 160–168.
Castelli F., Contillo R. and Miceli F. 1996. Non-destructive determination of leaf chlorophyll content in four crop species. Journal of Agronomy & Crop Science 177: 275–283.
Chapin F.S., Autumn K. and Pugnaire F. 1993. Evolution of suites of traits in response to environmental-stress. American Naturalist 142S: S78–S92.
Coomes D.A. and Grubb P.J. 2000. Impacts of root competition in forests and woodlands: a theoretical framework and review of experiments. Ecological Monographs 70: 171–207.
de Lillis M. and Fontanella A. 1992. Comparative phenology and growth in different species of the Mediterranean maquis of central Italy. Vegetatio 99-100: 83–96.
Eissenstat D.M. 1992. Costs and benefits of constructing roots of small diameter. Journal of Plant Nutrition 15: 763–782.
Eliáš P. and Masarovi?ová E. 1986. Seasonal changes in leaf chlorophyll content of Mercurialis perennis growing in deciduous and coniferous forests. Photosynthetica 20: 181–186.
Ellsworth D.S. and Reich P.B. 1992. Water relations and gas exchange of Acer saccharum seedlings in contrasting natural light and water regimes. Tree Physiology 10: 1–20.
Evans G.C. 1972. The Quantitative Analysis of Plant Growth. Blackwell Scientific, Oxford.
Evans J.R. 1989. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 78: 9–19.
Evans J.R. 1998. Photosynthetic characteristics of fast-and slowgrowing species. In: Lambers H., Poorter H. and Van Vuuren M.M.I. (eds), Inherent Variation in Plant Growth. Backhuys Publishers, Leiden, the Netherlands, pp. 101–120.
Evans J.R. and Poorter H. 2001. Photosynthetic acclimation of plants to growth irradiance: the relative importance of specific leaf area and nitrogen partitioning in maximizing carbon gain. Plant, Cell and Environment 24: 755–767.
Fanizza G., Ricciardi L. and Bagnulo C. 1991. Leaf greenness measurements to evaluate water stressed genotypes in Vitis vinifera. Euphytica 55: 27–31.
Fearnside P.M. 1995. Potential impacts of climatic change on natural forests and forestry in Brazilian Amazonia. Forest Ecology and Management 78: 51–70.
Field C. and Mooney H.A. 1986. The photosynthesis-nitrogen relationship in wild plants. In: Givnish T.J. (ed.), On the Economy of Plant Form and Function. Cambridge University Press, Cambridge, pp. 25–56.
Fitter A.H. and Stickland T.R. 1991. Architectural analysis of plant root systems. 2. Influence of nutrient supply on architecture in contrasting plant species. New Phytologist 118: 383–389.
Gilligan C.A. 1986. Use and misuse of the analysis of variance in plant pathology. Advances in Plant Pathology 5: 225–261.
Givnish T.J. 1988. Adaptation to sun and shade: a whole-plant perspective. Australian Journal of Plant Physiology 15: 63–92.
Gratani L. 1992. A non-destructive method to determine chlorophyll content of leaves. Photosynthetica 26: 469–473.
Gratani L. 1997. Canopy structure, vertical radiation profile and photosynthetic function in a Quercus ilex evergreen forest. Photosynthetica 33: 139–149.
Gratani L., Marzi P. and Crescente M.F. 1992. Morphological adaptions of Quercus ilex leaves in the Castelporziano forest. Vegetatio 99-100: 155–161.
Grier C.C. and Running S.W. 1977. Leaf area of mature northwestern coniferous forests: relation to site water balance. Ecology 58: 893–899.
Grime J.P. 1966. Shade avoidance and shade tolerance in flowering plants. In: Bainbridge R., Evans G.C. and Rackham O. (eds), Light as an Ecological Factor. Blackwell Scientific, Oxford, pp. 187–207.
Grime J.P. 1979. Plant Strategies and Vegetation Processes. Wiley, Chichester.
Grubb P.J. 1984. Some growth points in investigative plant ecology.In: Cooley J.H. and Golley F.B. (eds), Trends in Ecological Research for the 1980s. Plenum, New York, pp. 51–74.
Grubb P.J. 1998. A reassessment of the strategies of plants which cope with shortages of resources. Perspectives in Plant Ecology, Evolution and Systematics 1: 3–31.
Grubb P.J., Grubb E.A.A. and Miyata I.1975.Leaf structure and function in evergreen trees and shrubs of Japanese warm temperate rain-forest. 1. Structure of lamina. Botanical Magazine-Tokyo 88: 197–211.
Grubb P.J., Turner I.M. and Burslem D.F.R.P. 1994. Mineral nutrient status of coastal hill dipterocarp forest and adinandra belukar in Singapore: analysis of soil, leaves and litter. Journal of Tropical Ecology 10: 559–577.
Grubb P.J., Lee W.G., Kollman J. and Wilson J.B. 1996. Interaction of irradiance and soil nutrient supply on growth of seeedlings of ten European tall-shrub species and Fagus sylvatica. J. Ecol. 84: 827–840.
Herrera C.M. 1987. Vertebrate-dispersed plants of the Iberian peninsula: a study of fruit characteristics. Ecological Monographs 57: 305–331.
Herrera C.M., Jordano P., Lopez-Soria L. and Amat J.A. 1994. Recruitment of a mast-fruiting, bird-dispersed tree – bridging frugivore activity and seedling establishment. Ecological Monographs 64: 315–344.
Hunt R. and Cornelissen J.H.C. 1997. Components of relative growth rate and their interrelations in 59 temperate plant species. New Phytologist 135: 395–417.
Karl T.R., Knight R.W. and Plummer N. 1995. Trends in high-frequency climate variability in the twentieth century. Nature 377: 217–220.
Kerstiens G. 1996. Cuticular water permeability and its physiological significance. Journal of Experimental Botany 47: 1813–1832.
Kohyama T. and Grubb P.J. 1994.Below-and above-ground allometries of shade-tolerant seedlings in a Japanese warm-temperate rain forest. Functional Ecology 8: 229–236.
Kull O. and Niinemets U. 1998. Distribution of leaf photosynthetic properties in tree canopies: comparison of species with different shade tolerance. Functional Ecology 12: 472–479.
Latham P.E. 1992. Co-occurring tree species change rank in seedlings performance with resources varied experimentally. Ecology 73: 2129–2144.
Levitt J. 1980. Responses of Plants to Environmental Stresses. II.Water, Radiation, Salt, and Other Stresses. Academic Press, New York.
Lichtenthaler H.K. 1985. Differences in morphology and chemical composition of leaves grown at different light intensities and qualities. In: Baker N.R., Davies W.J. and Ong C.K. (eds), Control of Leaf Growth. Cambridge University Press, Cambridge, pp. 201–221.
Loach K. 1970. Shade tolerance of tree seedlings. II. Growth analysis of plants raised under artificial shade. New Phytologist 69: 273–286.
Lopez B., Sabate S. and Gracia C. 1998. Fine root dynamics in a Mediterranean forest: effects of drought and stem density. Tree Physiology 18: 601–606.
Lovett Doust L. 1981. Population dynamics and local specialization in a clonal perennial (Ranunculus repens). I. The dynamics of ramets in contrasting habitats. Journal of Ecology 69: 757–768.
Ludlow M.M. and Powles S.B. 1988. Effects of photoinhibition induced by water stress on growth and yield of grain sorghum. Australian Journal of Plant Physiology 15: 179–194.
Lusk C.H. and Reich P.B. 2000. Relationships of leaf dark respiration with light environment and tissue nitrogen content in juveniles of 11 cold-temperate tree species. Oecologia 123: 318–329.
Manetas Y., Grammatikopoulos G. and Kyparissis A. 1998. The use of the portable, non-destructive, SPAD-502 (Minolta) chlorophyll meter with leaves of varying trichome density and anthocyanin content. Journal of Plant Physiology 153: 513–516.
Marañón T. and Grubb P.J. 1993. Physiological basis and ecological significance of the seed size and relative growth rate relationship in Mediterranean annuals. Functional Ecology 7: 591–599.
Marquard R.D. and Tipton J.L. 1987. Relationship between extractable chlorophyll and an in situ method to estimate leaf greenness. HortScience 22.
Monk C. 1966. Ecological importance of root/shoot ratios. Bulletin of the Torrey Botanical Club 93: 402–406.
Muraoka H., Tang Y., Koizumi H. and Washitani I. 1997. Combined effects of light and water availability on photosynthesis and growth of Arisaema heterophyllum in the forest understory and an open site. Oecologia 112: 26–34.
Murchie E.H. and Horton P. 1997. Acclimation of photosynthesis to irradiance and spectral quality in British plant species: chlorophyll content, photosynthetic capacity and habitat preference. Plant, Cell and Environment 20: 438–448.
Navas M.-L. and Garnier E. 1990. Demography and growth forms of the clonal perennial Rubia peregrina in Mediterranean vineyard and unmanaged habitats. Journal of Ecology 78: 691–712.
Niinemets U. and Kull K. 1994. Leaf weight per area and leaf size of 85 Estonian woody species in relation to shade tolerance and light availability. Forest Ecology and Management 70: 1–10.
Niklas K.J. 1994. Plant Allometry. University of Chicago Press, Chicago.
Ojeda F., Arroyo J. and Marañón T. 1995. Biodiversity components and conservation of Mediterranean heathlands in southern Spain. Biological Conservation 72: 61–72.
Packham J.R. and Willis A.J. 1977. Effects of shading on Oxalis acetosella. Journal of Ecology 65: 619–642.
Packham J.R. and Willis A.J. 1982. Effects of shading and soil on Galeobdolon luteum. Journal of Ecology 70: 491–512.
Pallardy S.G. and Rhoads J.L. 1993. Morphological adaptations to drought in seedlings of deciduous angiosperms. Canadian Journal of Forest Research 23: 1766–1774.
Pearsall W.H. 1927. Growth studies VI. On the relative sizes of growing plant organs. Annals of Botany 41: 549–559.
Peat H.J. and Fitter A.H. 1994. A comparative study of the distribution and density of stomata in the British flora. Biological Journal of the Linnean Society 52: 377–393.
Peñuelas J., Filella I., Llusia J., Siscart D. and Piñol J. 1998. Comparative field study of spring and summer leaf gas exchange and photobiology of the mediterranean trees Quercus ilex and Phillyrea latifolia. Journal of Experimental Botany 49: 229–238.
Pisek A. and Berger E. 1938.Kutikulare Transpiration und Trockenresistenz isolierter Blätter und Sprosse. Planta 28: 124–155.
Poorter L. and Hayashida-Oliver Y. 2000. Effects of seasonal drought on gap and understorey seedlings in a Bolivian moist forest. Journal of Tropical Ecology 16: 481–498.
Rayner J.M.V. 1985. Linear relations in biomechanics: the statistics of scaling functions. Journal of the Zoological Society of London A206: 415–439.
Reich P.B., Uhl C., Walters M.B. and Ellsworth D.S. 1991. Leaf lifespan as a determinant of leaf structure and function among 23 tree species in Amazonian forest communities. Oecologia 86: 16–24.
Reich P.B., Ellsworth D.S., Walters M.B., Vose J.M., Gresham C., Volin J.C. et al. 1999. Generality of leaf trait relationships: a test across six biomes. Ecology 80: 1955–1969.
Rice W.R. 1989. Analyzing tables of statistical tests. Evolution 43: 223–225.
Ricker W.E. 1984. Computation and uses of central trend lines. Canadian Journal of Zoology 62: 1897–1905.
Ricklefs R.E. and Matthew K.K. 1982. Chemical characteristics of the foliage of some deciduous trees in southeastern Ontario. Canadian Journal of Botany 60: 2037–2045.
Roderick M.L., Berry S.L., Noble I.R. and Farquhar G.D. 1999a. A theoretical approach to linking the composition and morphology with the function of leaves. Functional Ecology 13: 683–695.
Roderick M.L., Berry S.L., Saunders A.R. and Noble I.R. 1999b. On the relationship between the composition, morphology and function of leaves. Functional Ecology 13: 696–710.
Sack L. and Grubb P.J. 2001. Why do species of woody seedlings change rank in relative growth rate between low and high irradiance? Functional Ecology 15: 145–154.
Sack L. and Grubb P.J. 2002. The combined impacts of deep shade and drought on the growth and biomass allocation of shade tolerant woody seedlings. Oecologia 131: 175–185.
Sala A. 1999. Modelling canopy gas exchange during summer drought. In: Roda F., Retana J., Gracia C.A. and Bellot J. (eds), Ecology of Mediterranean Evergreen Forests. Springer, Berlin, pp. 149–162.
Sala A., Sabate S., Gracia C. and Tenhunen J.D. 1994. Canopy structure within a Quercus ilex forested watershed – variations due to location, phenological development, and water availability. Trees 8: 254–261.
Salisbury E.J. 1927. On the causes and ecological significance of stomatal frequency, with special reference to the woodland flora. Philosophical Transactions of the Royal Society of London B216: 1–65.
Save R., Castell C. and Terradas J. 1999. Gas exchange and water relations. In: Roda F., Retana J., Gracia C.A. and Bellot J. (eds), Ecology of Mediterranean Evergreen Forests. Springer, Berlin, pp. 135–148.
Schreiber L. and Riederer M. 1996. Ecophysiology of cuticular transpiration: comparative investigation of cuticular water permeability of plant species from different habitats. Oecologia 107: 426–432.
Seemann J.R., Sharkey T.D., Wang J. and Osmond C.B. 1987. Environmental effects on photosynthesis, nitrogen-use efficiency, and metabolite pools in leaves of sun and shade plants. Plant Physiology 84: 796–802.
Shipley B. 1995. Structured interspecific determinants of specific leaf area in 34 species of herbaceous angiosperms. Functional Ecology 9: 312–319.
Singha S. and Townsend E.C. 1989. Relationship between chromaticity values and chlorophyll concentration in apple, grape and peach leaves. HortScience 24.
Siwecki R. and Ufnalski K. 1998. Review of oak stand decline with special reference to the role of drought in Poland. European Journal of Forest Pathology 28: 99–112.
Small E. 1972. Photosynthetic rates in relation to nitrogen cycling as an adaptation to nutrient deficiency in peat bog plants. Canadian Journal of Botany 50: 2227–2233.
Smith T. and Huston M. 1989. A theory of the spatial and temporal dynamics of plant communities. Vegetatio 83: 49–69.
Snedecor G.W. and Cochran W.G. 1989. Statistical Methods. 8th edn. Iowa State University Press, Ames.
Sokal R.R. and Rohlf F.J. 1995. Biometry. 3rd edn. Freeman, New York.
Specht R.L. and Specht A. 1989. Canopy structure in Eucalyptusdominated communities in Australia along climatic gradients. Acta Oecologica 10: 191–213.
Stewart G.R., Gracia C.A., Hegarty E.E. and Specht R.L. 1990. Nitrate reductase activity and chlorophyll content in sun leaves of subtropical Australian closed-forest (rainforest) and openforested communities. Oecologia 82: 544–551.
Stuefer J.F. 1998. Two types of division of labour in clonal plants: benefits, costs and constraints. Perspectives in Plant Ecology, Evolution and Systematics 1: 47–60.
Turner I.M., Lucas P.W., Becker P., Wong S.C., Yong J.W.H., Choong M.F. et al. 2000. Tree leaf form in Brunei: a heath forest and a mixed dipterocarp forest compared. Biotropica 32: 53–61.
Tutin T.G., Heywood V.H., Burges N.A., Valentine D.H., Walters S.M. and Webb D.A. 1964–1980. Flora Europaea. Vol. 1–5. Cambridge University Press, Cambridge.
Valladares F. and Pearcy R.W. 1997. Interactions between water stress, sun-shade acclimation, heat tolerance and photoinhibition in the sclerophyll Heteromeles arbutifolia. Plant, Cell and Environment 20: 25–36.
Vance N.C. and Zaerr J.B. 1991. Influence of drought stress and low irradiance on plant water relations and structural constituents in needles of Pinus ponderosa seedlings. Tree Physiology 8: 175–184.
Veenendaal E.M., Swaine M.D., Agyeman V.K., Blay D., Abebrese I.K. and Mullins C.E. 1995. Differences in plant and soil water relations in and around a forest gap in West Africa during the dry season may influence seedling establishment and survival. Journal of Ecology 84: 83–90.
Veneklaas E.J. and Poorter L. 1998. Growth and carbon partitioning of tropical tree seedlings in contrasting light environments. In: Lambers H., Poorter H. and Van Vuuren M.M.I. (eds), Inherent Variation in Plant Growth. Backhuys Publishers, Leiden, The Netherlands, pp. 337–361.
von Willert D.J., Eller B.M., Werger M.J.A. and Brinckmann E. 1990. Desert succulents and their life strategies. Vegetatio 90: 133–143.
Walters M.B. and Reich P.B. 1996. Are shade tolerance, survival, and growth linked? Low light and nitrogen effects on hardwood seedlings. Ecology 77: 841–853.
Walters M.B. and Reich P.B. 1999. Low-light carbon balance and shade tolerance in the seedlings of woody plants: do winter-deciduous and broad-leaved evergreen species differ? New Phytologist 143: 143–154.
Wright S.J., Machado J.L., Mulkey S.S. and Smith A.P. 1992. Drought acclimation among tropical forest shrubs (Psychotria, Rubiaceae). Oecologia 89: 457–463.
Yamada T., Yamakura T. and Lee H.S. 2000. Architectural and allometric differences among Scaphium species are related to microhabitat preferences. Functional Ecology 14: 731–737.
Zahner R. 1955. Soil water depletion by pine and hardwood stands during a dry season. Forest Science 1: 258–264.
Zar J.H. 1999. Biostatistical Analysis. 4th edn. Prentice-Hall, Upper Saddle River, New Jersey.
Zavala M.A., Espelta J.M. and Retana J. 2000. Constraints and trade-offs in Mediterranean plant communities: the case of holm oak-aleppo pine forests. Botanical Review 66: 119–149.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sack, L., Grubb, P.J. & Marañón, T. The functional morphology of juvenile plants tolerant of strong summer drought in shaded forest understories in southern Spain. Plant Ecology 168, 139–163 (2003). https://doi.org/10.1023/A:1024423820136
Issue Date:
DOI: https://doi.org/10.1023/A:1024423820136