Abstract
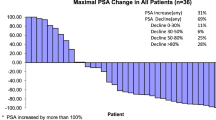
A single centre phase II study wasconducted to determine the toxicity andactivity of CaelyxTM in hormone refractorymetastatic prostate cancer. Doxorubicinis known to be active in this setting andliposomal encapsulation may enhance itstherapeutic efficacy and also reducetoxicity. Fourteen patients with hormonerefractory metastatic prostate cancer weretreated with CaelyxTM 50 mg/m2 onceevery four weeks. All patients hadradiologically proven bone metastases andthree also had soft tissue metastaticdisease. All patients were evaluable fortoxicity and response was assessable inthirteen cases. Three PSA responses weredocumented in patients with non-measurabledisease. No patient had an objectiveresponse in measurable disease. Thecommonest toxicity was cutaneous and thiswas dose limiting in two patients. Gastrointestinal upset was frequent butgenerally mild. One patient died shortlyafter an episode of neutropaenic sepsiswith associated grade 3 mucositis followinghis third cycle of chemotherapy. Weconfirmed the toxicity profile of CaelyxTMbut its modest antitumour efficacy in thisgroup of patients suggests little promisefor future study in metastatic prostatecancer.
Similar content being viewed by others
References
Scottish Cancer Intelligence Unit: Trends in cancer survival in Scotland 1971–1995. Information & Statistics Division, National Health Service in Scotland, Edinburgh, 2000, p112
Oh WK, Kantoff PW: Management of hormone refractory prostate cancer: current standards and future prospects. J Urol 160: 1220–1229, 1998
Yagoda A, Petrylak D: Cytotoxic chemotherapy for advanced hormone-resistant prostate cancer. Cancer 71:S1098–1109, 1993
Tannock IF, Osoba D, Stockler MR, Ernst DS, Neville AJ, Moore MJ, Armitage GR, Wilson JJ, Venner PM, Coppin CM, Murphy KC: Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J Clin Oncol 14:1756–1764, 1996
Northfelt DW, Dezube BJ, Thommes JA, Miller BJ, Fischl MA, Friedman-Kien A, Kaplan LD, Du Mond C, Mamelok RD, Henry DH: Pegylated-liposomal doxorubicin versus doxorubicin, bleomycin and vincristine in the treatment of AIDS-related Kaposi's sarcoma: results of a randomised phase III clinical trial. J Clin Oncol 16:2445–2551, 1998
Ranson MR, Carmichael J, O'Byrne K, Stewart S, Smith D, Howell A: Treatment of advanced breast cancer with sterically stabilised liposomal doxorubicin: results of a multicentre phase II trial. J Clin Oncol 15:3185–3191, 1997
Markman M, Kennedy A, Webster K, Peterson G, Kulp B, Belinson J: Phase II trial of liposomal doxorubicin (40 mg/m2) in platinum/paclitaxel-refractory ovarian and fallopian tube cancers and primary carcinoma of the peritoneum. Gynaecol Oncol 78:369–372, 2000
Vaage J, Barberá-Guillem E, Abra R, Huang A, Working P: Tissue distribution and therapeutic effect of intravenous free or encapsulated doxorubicin on human prostate carcinoma xenografts. Cancer 73:1478–1484, 1994
Hubert A, Lyass O, Pode D, Gabizon A: Doxil (Caelyx): an exploratory study with pharmacokinetics in patients with hormone-refractory prostate cancer. Anticancer Drugs 11:123–127, 2000.
Bubley GJ, Carducci M, Dahut W, Dawson N, Daliani D, Eisenberger M, Figg WD, Friedlin B, Halabi S, Hudes G, Hussain M, Kaplan R, Myers C, Oh W, Petrylak DP, Reed E, Roth B, Sartor O, Scher H, Simons J, Sinibaldi V, Small EJ, Smith MR, Trump DL, Vollmer R, Wilding G: Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol 17:3461–3467, 1999
Aaronson NK, Ahmedazi S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC: The European Organisation for Research and Treatment of Cancer QLQ-Q30: a quality of life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85:365–376, 1993
Pollen JJ, Witzum KF, Ashburn WL: The flare phenomenon on radionucleotide bone scan in metastatic prostate cancer. Am J Roentgenol 142:773–336, 1984
Kelly WK, Sher HI, Mazumdar M, Vlamis V, Shwartz M, Fossa SD: Prostate-specific antigen as a measure of disease outcome in metastatic hormone refractory prostate cancer. J Clin Oncol 11:607–615, 1993
Garcia AA, Kempf RA, Rogers M, Muggia FM: A phase II study of Doxil (liposomal doxorubicin): Lack of activity in poor prognosis soft tissue sarcomas. Ann Oncol 9:1131–1133, 1998
Muggia FM, Hainsworth JD, Jeffers S, Miller P, Groshen S, Tan M, Roman L, Uziely B, Muderspach L, Garcia A, Burnett A, Greco FA, Morrow CP, Paradiso LJ, Lian LJ: Phase II study of liposomal doxorubicin in refractory ovarian cancer: antitumor activity and toxicity modification by lipsomal encapsulation. J Clin Oncol 15:987–993, 1997
Hamilton A, Coleman R, Mauriac L, Awada A, Nooij M, Piccart M, Van Vrecken A, Bruning P: Phase I study of Caelyx at a six week interval in patients with metastatic breast cancer. Breast Cancer Res Treat 50(3):324, abstract 535, 1998
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
McMenemin, R., Macdonald, G., Moffat, L. et al. A Phase II Study of CaelyxTM (Liposomal Doxorubicin) in Metastatic Carcinoma of the Prostate: Tolerability and Efficacy Modification by Liposomal Encapsulation. Invest New Drugs 20, 331–337 (2002). https://doi.org/10.1023/A:1016225024121
Issue Date:
DOI: https://doi.org/10.1023/A:1016225024121