Abstract
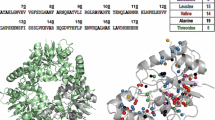
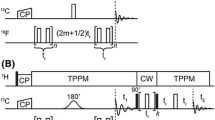
This report describes a novel NMR approach for mapping the interaction surface between an unlabeled ligand and a 13C,15N-labeled protein. The method relies on the spin inversion properties of the dipolar relaxation pathways and records the differential relaxation of two spin modes, where ligand and protein 1H magnetizations are aligned either in a parallel or anti-parallel manner. Selective inversion of protein protons is achieved in a straightforward manner by exploiting the one-bond heteronuclear scalar couplings (1JCH, 1JNH). Suppression of indirect relaxation pathways mediated by bulk water or rapidly exchanging protons is achieved by selective inversion of the water signal in the middle of the NOESY mixing period. The method does not require deuteration of the protein or well separated spectral regions for the protein and the ligand, respectively. Additionally, in contrast to previous methods, the new experiment identifies side-chain enzyme ligand interactions along the intermolecular binding interface. The method is demonstrated with an application to the B12-binding subunit of glutamate mutase from Clostridium tetanomorphum for which NMR chemical shift changes upon B12-nucleotide loop binding and a high-resolution solution structure are available.
Similar content being viewed by others
References
Barker, H.A., Weissbach, H. and Smith, R.D. (1958) Proc. Natl. Acad. Sci. USA, 44, 1093-1097.
Bartels, G., Nussberger, R., Kreppelt, F., Schreiber, J. and Eschenmoser, A., unpublished, see Kreppelt, F. (1991) Thesis, ETH-Zürich, No. 9458.
Breeze, A. (2000) Prog. NMR Spectrosc., 36, 323-372.
Clore, G.M. (2000) Proc. Natl. Acad. Sci. USA, 97, 9021-9025.
Chen, Y., Reizer, J., Saier, Jr., M.H., Fairbrother, W.J. and Wright, P.E. (1993) Biochemistry, 32, 32-37.
Dalvit, C., Ramage, P. and Hommel, U. (1998) J. Magn. Reson., 131, 148-153.
Dalvit, C., Cottens, S., Ramage, P. and Hommel, U. (1999) J. Biomol. NMR, 13, 43-50.
Delaglio, F., Grzesiek, S., Vuister, G.W., Zhu, G., Pfeifer, J. and Bax, A. (1995) J. Biomol. NMR, 6, 277-293.
Drennan, C.L., Huang, S., Drummond, J.T., Matthews, R.G. and Ludwig, M.L. (1994a) Science, 266, 1669-1674.
Drennan, C.L., Matthews, R.G. and Ludwig, M.L. (1994b) Curr. Opin. Struct. Biol., 4, 919-929.
Emerson, S.D., Madison, V.S., Palermo, R.E., Waugh, D.S., Scheffler, J.E., Tsao, K.-L., Kiefer, S.E., Liu, S.P. and Fry, D.C. (1995) Biochemistry, 34, 6911-6918.
Eschenmoser, A. (1988) Angew. Chem. Int. Ed. Engl., 100, 5-40.
Farmer, B.T., Constantine, K.L., Goldfarb, V., Friedrichs, M.S., Wittekind, M., Yanchunas, J., Robertson, J.G. and Mueller, L. (1996) Nat. Struct. Biol., 3, 995-997.
Foster, M.P., Wuttke, D.S., Clemens, K.R., Jahnke, W., Rahakrishnan, I., Tennant, L., Reymond, M., Chung, J. and Wright, P.E. (1998) J. Biomol. NMR, 12, 51-71.
Garrett, D.S., Seok, Y.-J., Peterkofsky, P., Clore, G.M. and Gronenborn, A.M. (1997) Biochemistry, 26, 4393-4398.
Geen, H. and Freeman, R. (1991) J. Magn. Reson., 93, 93-141.
Gemmecker, G., Olejniczak, E.T. and Fesik, S.W. (1992) J. Magn. Reson., 96, 199-204.
Gronenborn, A.M. and Clore, G.M. (1993) J. Mol. Biol., 233, 331-335.
Grzesiek, S., Bax, A., Clore, G.M., Gronenborn, A.M., Hu, J.S., Kaufman, J., Palmer, I., Stahl, S.J. and Wingfield, P.T. (1996) Nat. Struct. Biol., 3, 340-345.
Hajduk, P.J., Augeri, D.J., Mack, J., Mendoza, R., Yang, J., Betz, S.F. and Fesik, S.W. (2000) J. Am. Chem. Soc., 122, 7898-7904.
Holloway, D.E. and Marsh, E.N.G. (1994) J. Biol. Chem., 269, 20425-20430.
Ikura, M. and Bax, A. (1992) J. Am. Chem. Soc., 114, 2433-2440.
Janin, J., Miller, S. and Chothia, C. (1988) J. Mol. Biol., 204, 155-164.
Johnson, B.A. and Blevins, R.A. (1994) J. Biomol. NMR, 4, 603-614.
Koradi, R., Billeter, M. and Wüthrich, K. (1996) J. Mol. Graphics, 14, 51-55.
Kupce, E. and Freeman, R. (1995) J. Magn. Reson., A115, 273-276.
Lepre, C.A., Thomson, J.A. and Moore, J.M. (1992) FEBS Lett., 302, 89-96.
Lee, W., Revington, M.J., Arrowsmith, C. and Kay, L.E. (1994) FEBS Lett., 350, 87-90.
Mancia, F., Keep, N.H., Nakagawa, A., Leadlay, P.F., McSweeney, S., Rasmussen, B., Bösecke, P., Diat, O. and Evans, P.R. (1996) Structure, 4, 339-350.
Marion, D., Ikura, M., Tschudin, R. and Bax, A. (1989) J. Magn. Reson., 85, 393-399.
Marsh, E.N.G. and Holloway, D.E. (1993) FEBS Lett., 317, 44-48.
McCoy, M. and Mueller, L. (1992) J. Am. Chem. Soc., 114, 2108-2112.
Neuhaus, D. and Williamson, M.P. (2000) The Nuclear Overhauser Effect in Structural and Conformational Analysis, Wiley-VCH, New York, NY.
Otting, G., Senn, H., Wagner, G. and Wüthrich, K. (1986) J. Magn. Reson., 85, 500-505.
Otting, G. and Wüthrich, K. (1990) Q. Rev. Biophys., 23, 39-96.
Petros, A.M., Kawai, M., Luly, J.R. and Fesik, S.W. (1992) FEBS Lett., 308, 309-314.
Ramos, A., Kelly, G., Hollingworth, D., Pastore, A. and Frenkiel, T. (2000) J. Am. Chem. Soc., 122, 11311-11314.
Reitzer, R., Gruber, K., Jogl, G., Wagner, U.G., Bothe, H., Buckel, W. and Kratky, C. (1999) Structure, 7, 891-902.
Rosen, M.K., Gardner, K.H., Willis, R.C., Parris, W.E., Pawson, T. and Kay, L.E. (1996) J. Mol. Biol., 263, 627-636.
Ross, A., Salzmann, M. and Senn, H. (1997) J. Biomol. NMR, 10, 389-396.
Sattler, M., Schleucher, J. and Griesinger, C. (1999) Prog. NMR Spectrosc., 34, 93-158.
Shuker, S.B., Hajduk, P.J., Meadows, R.P. and Fesik, S.W. (1996) Science, 274, 1531-1534.
Stuart, A.C., Borzilleri, K.A., Withka, J.M. and Palmer III, A.G. (1999) J. Am. Chem. Soc., 121, 5346-5347.
Takahashi, H., Nakanishi, T., Kami, K., Arata, Y. and Shimada, I. (2000) Nat. Struct. Biol., 7, 220-223.
Tollinger, M., Konrat, R., Hilbert, B.N., Marsh, E.N.G. and Kräutler, B. (1998) Structure, 6, 1021-1033.
van Nuland, N.A.J., Boelens, R., Scheek, R.M. and Robillard, G.T. (1995) J. Mol. Biol., 246, 180-193.
Wagner, G. and Wüthrich, K. (1979) J. Magn. Reson., 33, 675-680.
Wider, G., Weber, C. and Wüthrich, K. (1991) J. Am. Chem. Soc., 113, 4676-4678.
Zwahlen, C., Vincent, S.J.F., Di Bari, L., Levitt, M.H. and Bodenhausen, G. (1994) J. Am. Chem. Soc., 116, 362-368.
Zwahlen, C., Legault, P., Vincent, S.J.F., Greenblatt, J., Konrat, R. and Kay, L.E. (1997) J. Am. Chem. Soc., 119, 6711-6721.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Eichmüller, C., Tollinger, M., Kräutler, B. et al. Mapping the ligand binding site at protein side-chains in protein-ligand complexes through NOE difference spectroscopy. J Biomol NMR 20, 195–202 (2001). https://doi.org/10.1023/A:1011299009214
Issue Date:
DOI: https://doi.org/10.1023/A:1011299009214