Abstract
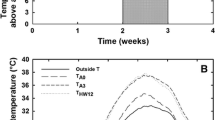
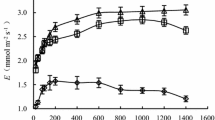
The interaction of extreme temperature events with future atmospheric CO2 concentrations may have strong impacts on physiological performance of desert shrub seedlings, which during the critical establishment phase often endure temperature extremes in conjunction with pronounced drought. To evaluate the interaction of drought and CO2 on photosynthesis during heat stress, one-year-old Larrea tridentata[DC] Cov. seedlings were exposed to nine days of heat with midday air temperature maxima reaching 53 °C under three atmospheric CO2 concentrations (360, 550 and 700 μmol mol−1) and two water regimes (well-watered and droughted). Photosynthetic gas exchange, chlorophyll fluorescence and water potential responses were measured prior to, during and one week following the high temperature stress event. Heat stress markedly decreased net photosynthetic rate (A net), stomatal conductance (g s), and the photochemical efficiency of photosystem II (F v/F m) in all plants except for well-watered L. tridentata grown in 700 μmol mol−1 CO2. A net and g s remained similar to pre-stress levels in these plants. In droughted L. tridentata, A net was ca. 2× (in 550 μmol mol−1 CO2) to 3× (in 700 μmol mol−1 CO2) higher than in ambient-CO2-grown plants, while g s and F v/F m were similar and low in all CO2 treatments. Following heat stress, g s in all well-watered plants rose dramatically, exceeding pre-stress levels by up to 100%. In droughted plants, g s and A net rose only in plants grown at elevated CO2 following release from heat. This recovery response was strongest at 700 μmol mol−1 CO2, which returned to A net and g s values similar to pre-heat following several days of recovery. Extreme heat diminished the photosynthetic down-regulation response to growth at elevated CO2 under well-watered conditions, similar to the action of drought. Ambient-CO2-grown L. tridentata did not show significant recovery of photosynthetic capacity (A \max and CE) after alleviation of temperature stress, especially when exposed to drought, while plants exposed to elevated CO2 appeared to be unaffected. These findings suggest that elevated CO2 could promote photosynthetic activity during critical periods of seedling establishment, and enhance the potential for L. tridentata to survive extreme high temperature events.
Similar content being viewed by others
References
BassiriRad, H., Reynolds, J. F., Virginia, R. A. & Brunelle, M. H. 1997. Growth and root NO3 −and PO3 −4 uptake capacity of three desert species in response to atmospheric CO2 enrichment. Austr. J. Plant Physiol. 24: 353–358.
Bassow, S. L., McConnaughay, K. D. M. & Bazzaz, F. A. 1994. The response of temperate tree seedlings grown in elevated CO2 to extreme temperature events. Ecol. Appl. 4: 593–603.
Chapin, F. S., Autumn, K. & Pugnaire, F. 1993. Evolution of suites of traits in response to environmental stress. Am. Nat. 142: S78–S92.
Chen, D. X., Coughenour, M. B., Eberts, D. & Thullen, J. S. 1994. Interactive effects of CO2 enrichment and temperature on the growth of dioecious Hydrilla verticellata. Env. Exp. Bot. 34: 345–353.
Coleman, J. S., Rocheforte, L., Bazzaz, F. A. & Woodward, F. I. 1991. Atmospheric CO2, plant nitrogen status, and the susceptibility of plants to an acute increase in temperature. Plant Cell Environ. 14: 667–674.
Field, C.B., Jackson, R.B. & Mooney, H.A. 1995. Stomatal responses to increased CO2: implications from the plant to the global scale. Plant Cell Environ. 18: 1214–1225.
Giardi, M.T., Cona, A., Geiken, B., Kucera, T., Masojidek, J. & Mattoo, A.K. 1996. Long-term drought stress induced structural and functional reorganization of photosystem II. Planta 199: 118–125.
Gibson, A.C. 1996. Structure- Function Relations of Warm Desert Plants. Springer-Verlag, Berlin.
Griffin, K.L. & Seemann, J.S. 1996. Plants, CO2 and photosynthesis in the 21st century. Chem. Biol. 3: 245–254.
Hamerlynck, E.P. & Knapp, A.K. 1996. Photosynthetic and stomatal responses to high temperature and light in two oaks at the western limit of their range. Tree Physiol. 16: 557–565.
Heckathorn, S.A., Poeller, G.J., Coleman, J.S. & Hallberg, R.L. 1996. Nitrogen availability alters patterns of accumulation of heat-stress induced proteins in plants. Oecologia 105: 413–418.
Hunt, H.W., Elliot, D.T., Detling, J.K., Morgan, J.A. & Chen, D.X. 1996. Responses of a C3 and a C4 perennial grass to elevated CO2 and temperature under different water regimes. Global Change Biol. 2: 35–47.
Huxman, K.A., Smith, S.D. & Neuman, D.S. 1999. Root hydraulic conductivity of Larrea tridentata and Helianthus annuus under elevated CO2. Plant Cell Environ. 22: 325–333.
Huxman, T.E., Hamerlynck, E.P., Moore, B.d., Smith, S.D., Jordan, D.N., Zitzer, S., Nowak, R.S., Coleman, J.S. & Seemann, J.R. 1998a. Photosynthetic down-regulation in Larrea tridentata exposed to elevated atmospheric CO2: interaction with drought under glasshouse and field (FACE) exposure. Plant Cell Environ. 21: 1153–1161.
Huxman, T.E., Hamerlynck, E.P., Loik, M.E. & Smith, S.D. 1998b. Gas exchange and chlorophyll fluorescence responses of three southwestern Yucca species to elevated CO2 and high temperature. Plant Cell Environ. 21: 1275–1283.
Hymus, G.J., Ellsworth D.D., Baker, N.R. & Long, S.P. 1999. Does free-air carbon dioxide enrichment affect photochemical energy use by evergreen trees in different seasons? A chlorophyll fluorescence study of mature loblolly pine. Plant Physiol. 120: 1183–1191.
Jacob, J., Greitner, C. & Drake, B.G. 1995. Acclimation of photosynthesis in relation to Rubisco and non-structural carbohydrate contents and in situ carboxylase activity in Scirpus olneyi grown at elevated CO2 in the field. Plant Cell Environ. 18: 875–884.
Jordan, P.W. & Nobel, P.S. 1979. Infrequent establishment of seedlings of Agave deserti in the northwestern Sonoran Desert. Am. J. Bot. 66: 1079–1084.
Knapp, A.K. 1992. Leaf gas exchange in Quercus macrocarpa (Fagaceae): rapid stomatal responses to variability in sunlight in a tree growth form. Am. J. Bot. 79: 599–604.
Knapp, A.K. & Smith,W.K. 1989. Influence of growth form on ecophysiological responses to variable sunlight in subalpine plants. Ecology 70: 1069–1082.
Knapp, A.K. & Smith, W.K. 1990. Contrasting stomatal responses to variable sunlight in two subalpine herbs. Am. J. Bot. 77: 226–231.
Knapp, A.K., Fahnestock, J.T. & Owensby C.E. 1994. Elevated CO2 alters stomatal responses to variable sunlight in the C4 grass, Andropogon gerardii. Plant Cell Environ. 17: 189–185.
Knapp, A.K., Hamerlynck, E.P., Ham, J.M. & Owensby, C.E. 1996. Responses in stomatal conductance to elevated CO2 in 12 grassland species that differ in growth form. Vegetatio 125: 31–41.
Kozlowski, T.T., Kramer, P.J. & Pallardy, S.G. 1991. The Physiological Ecology of Woody Plants. Academic Press, New York, NY.
Lewis, J.D., Tissue, D.T. & Strain, B.R. 1996. Seasonal response of photosynthesis to elevated CO2 in loblolly pine (Pinus taeda L.) over two growing seasons. Global Change Biol. 2: 103–114.
Loik, M. & Harte, J. 1996. High-temperature tolerance of Artemesia tridentata and Potentilla gracilis under a climate change manipulation. Oecologia 108: 224–231.
Long, S.P. 1991. Modification of the response of photosynthetic productivity to rising temperature by atmospheric CO2 concentrations: has its importance been underestimated? Plant Cell Environ. 14: 729–739.
Melillo, J.M., McGuire, A.D., Kicklighter, D.W., Moore, B. III, Vorosmarty, C.J. & Schloss, A.L. 1993. Global climate change and terrestrial net primary production. Nature 363: 234–240.
Meinzer, F.C., Wisdom, C.S., Gonzalez-Coloma, A., Rundel, P.W. & Shultz, L.M. 1990. Effects of leaf resin on stomatal behavior and gas exchange of Larrea tridentata. Funct. Ecol. 4: 579–584.
Mooney, H.A., Björkman, O. & Collatz, C.J. 1977. Photosynthetic Acclimation to Temperature andWater Stress in the Desert Shrub Larrea divaricata. Carnegie Inst.Washington Yearbook 76: 328–335.
Mooney, H.A., Björkman, O. & Collatz, C.J. 1978. Photosynthetic Acclimation to Temperature in the Desert Shrub, Larrea divaricata. I. Carbon dioxide exchange characteristics of intact leaves. Plant Physiol. 61: 406–410.
Morgan, J.A., Hunt, H.W., Monz, C.A. & LeCain, D.R. 1994. Consequences of growth at two carbon dioxide concentrations and two temperatures for leaf gas exchange in Pascopyrum smithii (C3) and Bouteloua gracilis (C4). Plant Cell Environ. 17: 1023–1033.
Nobel, P.S. 1991. Physicochemical and Environmental Plant Physiology. Academic Press, San Diego, CA.
Oechel, W.C., Strain, B.R. & Odening, W.R. 1972. Tissue water potential, photosynthesis, 14C-labeled photosynthate utilization and growth in the desert shrub Larrea divaricata Cav. Ecol. Monogr. 42: 127–141.
Oechel, W.C., Hastings, S.J., Vourlitis, Jenkins, M.A. & Hinkson, C.L. 1985. Direct effects of elevated CO2 in chaparral and Mediterranean-type ecosystems. Pp. 58–75. In: Moreno, J. & Oechel, W.C. (eds), Global Change and Mediterraneantype Ecosystems. Ecological Studies, Vol. 117, Springer-Verlag, Berlin.
Peters, R.L. 1992. Conservation of biological diversity in the face of climate change. Pp. 15–30. In: Peters, R.L. & Lovejoy T.E. (eds), Global Warming and Biological Diversity. Yale University Press, Yale.
Pockman T. & Sperry, J.S. 1997. Freezing induced xylem cavitation and the northern limit of Larrea tridentata. Oecologia 109: 19–27.
Read, J.J. & Morgan, J.J. 1996. Growth and partitioning in Pascopyrum smithii (C3) and Bouteloua gracilis (C4) as influenced by carbon dioxide and temperature. Annals Bot. 77: 487–496.
Rhoades, D.F. 1977. The antiherbivore chemistry of Larrea. Pp. 134–175. In: Mabry T.J., Hunziker J.H. & Difeo Jr., D.R. (eds), Creosote Bush: Biology and Chemistry of Larrea in New World Deserts, Dowden, Hutchinson and Ross, Inc, Stroudsburg, PA.
Roden, J.S. & Ball, M.C. 1996a. Growth and photosynthesis of two eucalypt species during high temperature stress under ambient and elevated [CO2]. Global Change Biol. 2: 115–128.
Roden, J.S. & Ball, M.C. 1996b. The effect of elevated [CO2] on growth and photosynthesis of two Eucalyptus species exposed to high temperatures and water deficits. Plant Physiol. 111: 909–919.
Rundel, P.W. & Gibson, A.C. 1996. Ecological Communities and Processes in a Mojave Desert Ecosystem. Cambridge University Press, Cambridge.
Sage, R.F. (1996) Atmospheric modification and vegetation response to environmental stress. Global Change Biol. 2: 79–83.
Seemann, J.R., Berry, J.A. & Downton, W.J.S. 1984. Photosynthetic response and adaptation to high temperatures in desert plants. Plant Physiol. 75: 364–368.
Sharkey, T.D. 1985. Photosynthesis in intact leaves of C3 plants: physics, physiology and rate limitations. Bot. Rev. 51: 53–105.
Smith, S.D. & Nowak, R.S. 1990. Ecophysiology of plants in the Intermountain lowlands. Pp. 179–241. In: Osmond C.B., Pitelka L.F. & Hidy G.F. (eds), Plant Biology of the Basin and Range. Springer-Verlag, Berlin.
Smith, S.D., Herr, C.A., Leary, K.L. & Piorkowski, J.M. 1995. Soil-plant water relations in a Mojave Desert mixed shrub community: A comparison of 3 geomorphic surfaces. J. Arid Environ. 29: 339–351.
Smith, S.D., Monson, R.K. & Anderson, J.E. 1997. Physiological Ecology of North American Desert Plants. Springer-Verlag, Berlin.
Smith, S.D., Jordan, D.N. & Hamerlynck, E.P. 1999. Effects of elevated CO2 and temperature stress on ecosystem processes. Pp. 107–137. In: Luo Y. & Mooney H.A. (eds), Carbon Dioxide and Environmental Stress, Academic Press, San Diego.
Stirling, C.M., Davey, P.A., Williams, T.G. & Long, S.P. 1997. Acclimation of photosynthesis to elevated CO2 and temperature in five British native species of contrasting functional type. Global Change Biol. 3: 237–246.
Wagner, D. 1996. Scenarios of extreme temperature events. Climatic Change 33: 385–407.
Watson, R.T., Rhode, H., Oescheger, H. & Sigenthaler, U. 1990. Greenhouse gases and aerosols. Pp. 1–40. In: Houghton J.T., Jenkins G.I. & Ephraums J.J. (eds), Climate Change: The IPCC Scientific Assessment, Cambridge University Press, Cambridge.
Wong, S.C., Cowan, I.R. & Farquhar, G.D. 1979. Stomatal conductance correlates with photosynthetic capacity. Nature 282: 424–426.
Von Caemmerer, S. & Farquhar, G.D. 1981. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153: 376–387.
Zar, J.H. 1974. Biostatistical Analysis. Prentice-Hall, Englewood Cliffs, NJ.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hamerlynck, E.P., Huxman, T.E., Loik, M.E. et al. Effects of extreme high temperature, drought and elevated CO2 on photosynthesis of the Mojave Desert evergreen shrub, Larrea tridentata. Plant Ecology 148, 183–193 (2000). https://doi.org/10.1023/A:1009896111405
Issue Date:
DOI: https://doi.org/10.1023/A:1009896111405