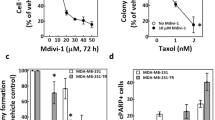
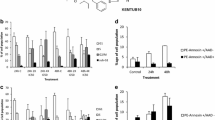
Abstract
TZT-1027, a newly synthesized dolastatin 10 derivative, is a potent antitumor agent which inhibits microtubule polymerization and perturbs microtubule dynamics. In this report, we investigated whether TZT-1027 inhibited the growth of various human cancer cells, and the cell death caused by TZT-1027 was due to apoptosis. In addition, we elucidated the apoptosis machinery induced by treatment with TZT-1027. The 50% growth-inhibitory concentrations (IC50 values) of TZT-1027 on cancer cells derived from various sources were not more than 5.9 ng/ml. TZT-1027 showed superior cytotoxicity than any other antitumor agents. Next, we evaluated morphological nuclear change, namely, chromatin condensation and DNA fragmentation. We used three cancer cell lines derived from different types in view of having apoptosis related protein, human leukemia HL-60 (in the presence of both Caspase-3 and Bcl-2), human breast cancer MCF-7 (in the absence of Caspase-3), and human prostate cancer DU145 (in the absence of Bcl-2). TZT-1027 induced DNA fragmentation in the presence but not absence of Caspase-3. Nevertheless, apoptic chromatin condensation was observed in all cancer cells even if there was no Caspase-3. Furthermore, we examined whether TZT-1027, microtubule-disrupting agent, influenced cell cycle progression. Flow cytometric analysis revealed the cells treated with TZT-1027, and with the other antimicrotubule agents, to be arrested at the G2/M phase and subsequently to show fragmented DNA smaller than that of G1 phase cells. Moreover, we tested TZT-1027 for its ability to induce Bcl-2 phosphorylation in human cancer cell lines. TZT-1027 and other agents which interacted with microtubules induced Bcl-2 phosphorylation, whereas DNA-damaging agents did not. The present results suggested an association of the growth-inhibitory effect of TZT-1027 with the induction of apoptosis and indicated that the apoptosis induced by TZT-1027 was followed by G2/M arrest even if there was no Caspase-3 or Bcl-2.
Similar content being viewed by others
References
Miyazaki K, Kobayashi M, Natsume T, et al. Synthesis and antitumor activity of novel dolastatin 10 analogs. Chem Pharm Bull 1995; 43: 1706–1718.
Pettit GR, Kamano Y, Herald CL, et al. The isolation and structure of a remarkable marine animal antineoplastic constituent: Dolastatin 10. J Am Chem Soc 1987; 109: 6883–6885.
Natsume T, Watanabe J, Fujio N, Miyasaka K, Kobayashi M. Characterization of the interaction of TZT-1027, a potent antitumor agent with tubulin. Jpn J Cancer Res 2000; 91: 737–747.
Kobayashi M, Natsume T, Tamaoki S, et al. Antitumor activity of TZT-1027, a novel dolasatatin 10 derivative. Jpn J Cancer Res 1997; 88: 316–327.
Nicolson DW, Ali A, Thornberry NA, et al. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature 1995; 376: 37–43.
Hengartner MO, Horvitz HR. C. elegans cell survival gene ced-9 encodes a functional homolog of the mammalian protooncogene bcl-2. Cell 1994; 76: 665–676.
Reed JC. Bcl-2 and regulation of programmed cell death. J Induction of apoptosis by TZT-1027 Cell Biol 1994; 124: 1–6.
Oltvai ZN, Korsmeyer SJ. Checkpoints of dueling dimers foil death wishes. Cell 1994; 79: 189–192.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays, J Immunol Methods 1983; 65: 55–63.
Crissman HA, Steinkamp JA. Rapid simultaneous measurement of DNA, protein, and cell volume in single cells from large mammalian cell populations. J Cell Biol 1973; 59: 766–771.
George P, Journey LJ, Goldstein MN. Effect of vincristine on the fine structure of HeLa cells during mitosis, J Natl Cancer Inst 1965; 35: 355–375.
Schiff PB, Horwitz SB. Taxol stabilizes microtubules in mouse fibroblast cells. Proc Natl Acad Sci USA 1980; 77: 1561–1565.
Rowinsky EK, Donehower RC, Jones RJ, Tucker RW. Microtubule changes and cytotoxicity in leukemic cell lines treated with taxol. Cancer Res 1988; 48: 4093–4100.
Halar S, Chintapalli J, Croce CM. Taxol induces bcl-2 phosphorylation and death of prostate cancer cells. Cancer Res 1996; 56: 1253–1255.
Berchem GJ, Bosseler M, Mine N, Avalosse B. Nanomolar range docetaxel treatment sensitizes MCF-7 cells to chemotherapy induced apoptosis, induces G2Marrest and phosphorylates bcl-2. Anticancer Res 1999; 19: 535–540.
Scatena CD, Stewart ZA, Mays D, et al. Mitotic phosphorylation of Bcl-2 during normal cell cycle progression and Taxolinduced growth arrest. J Biol Chem 1998; 273: 30777–30784.
Halder S, Basu A, Croce CM. Bcl-2 is the gurdian of microtubule integrity. Cancer Res 1997; 57: 229–233.
Janicke RU, Sprengart ML, Wati MR, Porter AG. Caspase-3 required for DNA fragmentation and morphological changes associated with apoptosis. J Biol Chem 1998; 273: 9357–9360.
Basu A, Halder S. Apoptosis, antiapoptosis and bcl-2. J Immunopharmacol 1996; 16: 41–47.
Halder S, Chintapalli J, Croce CM. Taxol induces bcl-2 phosphorylation and death of prostate cancer cells. Cancer Res 1996; 56: 1253–1255.
Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S. A caspase-activated DNase that degrates DNA during apoptosis, and its inhibitor ICAD. Nature 1998; 391: 43–50.
Liu X, Li P, Widlak P, et al. The 40 kDa subunit DNA fragmentation factor induces DNA fragmentation and chromatin condensation during apoptosis. Proc Natl Acad Sci USA 1998; 95: 8461–8466.
Ling Y-H, Tornos C, Perez-Soler R. Phosphorylation of Bcl-2 is a marker of M phase events and not a determinant apoptosis. J Biol Chem 1998; 273: 18984–18991.
Marianne SP, EmilyEW, Charles MR, Mikhail VB, Tito F. BclxL is phosphorylated in malignant cells following microtubule disruption. Cancer Res 1998; 58: 3331–3338.
Kobayashi M, Natsume T, Watanabe J, et al. Activity of a novel antitumor agent, TZT-1027. Folia Phamacol Jpn 1999; 114: 230–235.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Watanabe, J., Natsume, T., Fujio, N. et al. Induction of apoptosis in human cancer cells by TZT-1027, an antimicrotubule agent. Apoptosis 5, 345–353 (2000). https://doi.org/10.1023/A:1009687609330
Issue Date:
DOI: https://doi.org/10.1023/A:1009687609330