Abstract
Purpose: Understanding the causes of fertilization failure is an important research field in assisted reproductive programs. The present study aimed to evaluated the possible relationship between chromatin packaging quality (CMA3 staining) and (i) normal morphology and (ii) its ability to predict the functional integrity of spermatozoa in both in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) treatment programs.
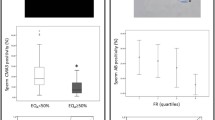
Methods: Semen of 140 men from IVF and ICSI couples were analyzed for sperm concentration, motility, morphology, and chromatin packaging (CMA3). For CMA3 classification, two cutoff values were used, namely, 44.5%±13 and 1 SD above the mean, i.e., 57.5% (rounded off to 60%). IVF and ICSI data were stratified using three basic cutoff values for CMA3 staining, namely, <44%, >44–60%, and >60%.
Results: Based on CMA3 results patients were divided into four groups, namely, group A, <44% CMA3 (n = 15, IVF); group B, ≥44% and <60% CMA3 (n = 39, IVF); group C, ≥60% CMA3 (n = 45 IVF); and group D, ≥60% CMA3(n = 41 ICSI). During receiver operator characteristic analyses the estimated cutoff value for CMA3 staining, to distinguish between <4% and ≥4% morphology groups, was 60%. The area under the curve was 0.89, sensitivity of 75%, and specificity of 100%. When IVF rates of >60% and <60% were used, the optimal CMA3 value for prediction of fertilization success again was recorded at 60%. The area under the curve was 0.76, sensitivity of 81.5%, and specificity of 63.6%.
Conclusions: Chromatin packaging assessments should be included as a complementary assay to the sequential diagnostic approach of the male-factor patients.
Similar content being viewed by others
REFERENCES
Van Steirteghem AC, Nagy Z, Joris H, Liu J, Staessen C, Smitz J, Wisanto A, Devroey P: High fertilization and implantation rates after intracytoplasmic sperm injection. Hum Reprod 1993;8:1061-1066
Kruger TF, Menkveld R, Stander FSH, Lombard CJ, van der Merwe JP, van Zyl JA, Smith K: Sperm morphologic features as a prognostic factor in in vitro fertilization. Fertil Steril 1986;46:1118-1123
Nagy ZP, Lui J, Cecile J, Silber S, Devroey P, Van Steirteghem A: Using ejaculated, fresh, and frozen-thawed epdidymal and testicular spermatozoa gives rise to comparable results after intracytoplasmic sperm injection. Fertil Steril 1995;808-815
Silber SJ, Van Steirteghem AC, Liu J, Nagy Z, Tournaye H, Devroey P: High fertilization and pregnancy rate after intracytoplasmic sperm injection with spermatozoa obtained from testicle biopsy. Hum Reprod 1995;10:148-152
Silber SJ, Nagy Z, Liu J, Tournaye H, Lissens W, Ferec C, Liebaers I, Devroey P, Van Steirteghem AC: The use of epididymal and testicular spermatozoa for intracytoplasmic sperm injection: The genetic implications for male infertility. Hum Reprod 1995;10:2031-2043
Kent-First M, Kol S, Muallem, A Blazer S, Itskovitz-Eldor J: Infertility in intracytoplasmic sperm injection derived sons. Lancet 1997;348:332-
De Jonge C. Attributes of fertile spermatozoa: An update. J Androl 1999;20:463-473
Consensus Workshop on Advanced Diagnostic Andrology Techniques: ESHRE Andrology Special Interest Group. Hum Reprod 1996;11:1463-1479
Pandiyan N, Jequier A: Mitotic chromosomal anomalies among 1210 infertile men. Hum Reprod 1996;11:2604-2608
Manicardi GC, Bianchi PG, Pantano S, Azzoni P, Bizzaro D, Bianchi U, Sakkas D: Presence of endogenous nicks in DNA of ejaculated human spermatozoa and its relationship to chromomycin A3 accessibility. Biol Reprod 1995;52:864-867
Sakkas D: The use of blastocyst culture to avoid inheritance of an abnormal paternal genome after ICSI. Hum Reprod 1999;14:4-5
Bianchi PG, Manicardi GC, Urner F, Campana A, Sakkas D: Chromatin packaging and morphology in ejaculated human spermatozoa: evidence of hidden anomalies in normal spermatozoa. Mol Hum Reprod 1996;2:139-144
Balhorn R: Mammalian protamine: Structures and molecular interactions. In Molecular Biology of Chromosome Function, KW Adolph (ed). New York, Springer Verlag pp 366-395
Franken DR, Franken CJ, de la Guerre H, de Villiers A: Normal sperm morphology and chromatin packaging: Comparison between analine blue and chromomycin A3 staining. Andrologia 1999;31:361-366
Granberg, M: Manual of Recommended Use of Scandinavian IVF Science Products, 2nd ed. 1999
World Health Organization. WHO Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction, 3rd ed. Cambridge, England, Cambridge University Press, 1992, p 23
Geigy scientific tables. In Physical Chemistry Composition of Blood Hematology, Somatometric Data, Vol 3, C Lentner (ed). Ciba Geigy, pp 58-59
Altman DG, Bland JM: Diagnostic tests, 3: Receiver operating characteristic plots. Br Med J 1994;309:188-
Schatten G, Hewittson L, Simerly C, Sutovsky P, Huszar G: Cell and molecular biological challenges of ICSI. ART before science? L Law Med Nas Ethics 1998;26:29-37
Sakkas D. Bianchi PG, Manicardi G, Bizzaro D, Bianchi U: Chromatin packaging anomalies and DNA damage in human sperm: Their possible implications in the treatment of male factor infertility. In Genetics of Human Male Fertility. C Barratt, C De Jonge, D Mortimer, J Parinaud (eds). EDK, 1997, pp 205-221
Corczyca W, Traganos F, Jesionowska H, Darzynkiewicz Z: Presence of DNA strand breaks and increased sensitivity of DNA in situ to denaturation in abnormal human sperm cells. Analogy to apoptosis of somatic cells. Exp Cell Res 1993;207:202-205
Bianchi PG, Manicardi GC, Bizzaro D, Bianchi U Sakkas D: Effect of DNA protamination on fluorochrome staining and in situ nick-translation of murine and human mature spermatozoa. Biol Reprod 1993;49:1038-1043
Evenson D, Jost L, Marshall D, et al.: Utility of sperm chromatin structure assay (SCSA) as adiagnostic and prognostic tool in the human fertility clinic. Hum Reprod 1999;14:1039-1049
Oehninger S, Acosta A, Veeck L, Brzyski R, Kruger TF, Muasher SJ, Hodgen GD: Recurrent failure of in vitro fertilization: Role of the hemizona assay in the sequential diagnosis of specific sperm-oocyte defects. Am J Obstet Gynecol 1991;164:1210-1215
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Esterhuizen, A.D., Franken, D.R., Lourens, J.G.H. et al. Chromatin Packaging as an Indicator of Human Sperm Dysfunction. J Assist Reprod Genet 17, 508–514 (2000). https://doi.org/10.1023/A:1009493824953
Issue Date:
DOI: https://doi.org/10.1023/A:1009493824953