Abstract
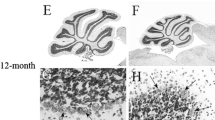
Qualitative and quantitative changes were found in the cerebellar circuitry of old as compared to young rats. The old group had a reduced number of synapses (at least 30%), however, there was an increase in the size of remaining synaptic components (13.5% for spine head volume, 66% for bouton volume, and 17% for the area of synaptic contact zones). Furthermore, there were pronounced morphological changes in the older group appearing as: 1) prominent lipofuscin bodies in Purkinje cell somata, 2) numerous myelinated fibers in the lower part of the molecular layer, 3) tortuous Purkinje cell dendrites in a thinned molecular layer, and 4) abundant vacuolar profiles and membrane swirls in small and intermediate-sized dendrites. Our findings suggest that Purkinje cell dendrites are dying-back reducing the target field for granule cells and that remaining synaptic sites compensate by increasing synaptic contact area as well as the size of pre- and postsynaptic structures.
Similar content being viewed by others
References
Bercombie, M. (1946) Estimation of nuclear populations from microtome sections. Anatomical Record 94, 239–247.
Adams, I. (1987a) Comparison of synaptic changes in the precentral and postcentral cerebral cortex of aging humans: a quantitative ultrastructural study. Neurobiology of Aging 8, 203–212.
Adams, I. (1987b) Plasticity of the synaptic contact zone following loss of synapses in the cerebral cortex of aging humans. Brain Research 424, 343–351.
Adams, I. & Jones, D. G. (1982) Synaptic remodelling and astrocytic hypertrophy in rat cerebral cortex from early to late adulthood. Neurobiology of Aging 3, 179–186.
Bakalian, A., Corman, B., Delhaye-Bouchaud, N. & Mariani, J. (1991) Quantitative analysis of the Purkinje cell population during extreme ageing in the cerebellum of theWistar=Louvain rat. Neurobiology of Aging 12, 425–430.
Bertoni-Freddari, C., Giuli, C., Pieri, C. & Paci, D. (1986a) Age-related morphological rearrangements of synaptic junctions in the rat cerebellum and hippocampus. Archives of Gerontology & Geriatrics 5, 297–304.
Bertoni-Freddari, C., Giuli, C., Pieri, C. & Paci, D. (1986b) Quantitative investigation of the morphological plasticity of synaptic junctions in rat dentate gyrus during aging. Brain Research 366, 187–192.
Brizzee, K. R. (1987) Neurons numbers and dendritic extent in normal aging and AlzheimerÕs disease. Neurobiology of Aging 8, 579–580.
Brizzee, K. R. & Ordy, J. M. (1979) Age pigments, cell loss and hippocampal function. Mechanisms of Ageing & Development 9, 143–162.
Brizzee, K. R., Ordy, J. M. & Bartus, R. T. (1980) Localization of cellular changes within multimodal sensory regions in aged monkey brain: possible implications for age-related cognitive loss. Neurobiology of Aging 1, 45–52.
Chalkley, H. W., Cornfiled, J. & Park, H. (1949) A method for estimating volume-surface ratios. Science 110, 295–297.
Chen, S. & Hillman, D. E. (1982) Plasticity of the parallel fiber-Purkinje cell synapse by spine takeover and new synapse formation in the adult rat. Brain Research 240, 205–220.
Chien, C. L., Mason, C. A. & Liem, R. K. (1996) Alpha-Internexin is the only neuronal intermediate filament expressed in developing cerebellar granule neurons. Journal of Neurobiogy 29, 304–318.
Coleman, P. D. & Flood, D. G. (1986) Dendritic proliferation in the aging brain as a compensatory repair mechanism. [Review]. Progress in Brain Research 70, 227–237.
Coleman, P. D. & Flood, D. G. (1987) Neuron numbers and dendritic extent in normal aging and AlzheimerÕs disease. [Review]. Neurobiology of Aging 8, 521–545.
Colon, E. J. (1972) The elderly brain. A quantitative analysis in the cerebral cortex of two cases. Psychiatria, Neurologia, Neurochirurgia 75, 261–270.
Cotman, C. W. & Scheff, S. W. (1979) Compensatory synapse growth in aged animals after neuronal death. Mechanisms of Ageing & Development 9, 103–117.
Cragg, B. G. (1975) The density of synapses and neurons in normal, mentally defective ageing human brains. Brain 98, 81–90.
Curcio, C. A. & Hinds, J. W. (1983) Stability of synaptic density and spine volume in dentate gyrus of aged rats. Neurobiology of Aging 4, 77–87.
Devaney, K. O. & Johnson, H. A. (1980) Neuron loss in the aging visual cortex of man. Journal of Gerontology 35, 836–841.
Dlugos, C. A. & Pentney, R. J. (1994) Morphometric analyses of Purkinje and granule cells in aging F344 rats. Neurobiology of Aging 15, 435–440.
Druge, H., Heinsen, H. & Heinsen, Y. L. (1986) Quantitative studies in ageing Chbb:THOM (Wistar) rats. II. Neuron numbers in lobules I, VIb + c and X. Bibliotheca Anatomica 28, 121–137.
Fliegner, K. H., Kaplan, M. P., Wood, T. L., Pintar, J. E. & Liem, R. K. (1994) Expression of the gene for the neuronal intermediate filament protein alpha-internexin coincides with the onset of neuronal differentiation in the developing rat nervous system. Journal of Comparative Neurology 342, 161–173.
Flood, D. G. & Coleman, P. D. (1988) Neuron numbers and sizes in aging brain: comparisons of human, monkey, and rodent data. [Review]. Neurobiology of Aging 9, 453–463.
Forbes, W. B. (1984) Aging-related morphological changes in the main olfactory bulb of the Fischer 344 rat. Neurobiology of Aging 5, 93–99.
Gall, C. & Lynch, G. (1978) Rapid axon sprouting in the neonatal rat hippocampus. Brain Research 153, 357–362.
Glick, R. & Bondareff, W. (1979) Loss of synapses in the cerebellar cortex of the senescent rat. Journal of Gerontology 34, 818–822.
Gundersen, H. J., Bagger, P., Bendtsen, T. F., Evans, S. M., Korbo, L., Marcussen, N. et al. (1988a) The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. Apmis 96, 857–881.
Gundersen, H. J., Bendtsen, T. F., Korbo, L., Marcussen, N., Moller, A., Nielsen, K. et al. (1988b) Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. Apmis 96, 379–394.
Hadj-Sahraoui, N., Frederic, F., Zanjani, H., Herrup, K., Delhaye-Bouchaud, N. & Mariani, J. (1997) Purkinje cell loss in heterozygous staggerer mutant mice during aging. Developmental Brain Research 98, 1–8.
Hall, T. C., Miller, K. H. & Corsellis, J. A. N. (1975) Variations in human Purkinje cell population according to age and sex. Neuropathology & Applied Neurobiology 1, 267–292.
Haug, H., Knebel, G., Mecke, E., Orun, C. & Sass, N. L. (1981) The aging of cortical cytoarchitectonics in the light of stereological investigations. Progress in Clinical & Biological Research 59B, 193–197.
Henderson, G., Tomlinson, B. E. & Gibson, P. H. (1980) Cell counts in human cerebral cortex in normal adults throughout life using an image analysing computer. Journal of Neurological Science 46, 113–136.
Hillman, D. & Chen, S. (1984a) Constraints on plasticity of cerebellar circuitry. In: Cerebellar Functions (edited by Bloedel, J. R., Dichgans J. & Precht, W.), pp. 300–317, Springer-Verlag.
Hillman, D. E. & Chen, S. (1984b) Reciprocal relationship between size of postsynaptic densities and their number: constancy in contact area. Brain Research 295, 325–343.
Hillman, D. & Chen, S. (1985a) Plasticity in the size of pre-and postsynaptic membrane specializations. In: Synaptic Plasticity and Remodeling (edited by Cotman, C.), pp. 39–76. New York: Guilford Publ.
Hillman, D. E. & Chen, S. (1985b) Compensation in the number of presynaptic dense projections and synaptic vesicles in remaining parallel fibres following cerebellar lesions. Journal of Neurocytology 14, 673–687.
Hinds, J. W. & Mcnelly, N. A. (1977) Aging of the rat olfactory bulb: growth and atrophy of constituent layers and changes in size and number of mitral cells. Journal of Comparative Neurology 72, 345–367.
Hinds, J. W. & Mcnelly, N. A. (1978) Dispersion of cisternae of rough endoplasmic reticulum in aging CNS neurons: a strictly linear trend. American Journal of Anatomy 152, 433–439.
Levine, M. S., Adinolfi, A. M., Fisher, R. S., Hull, C. D., Buchwald, N. A. & Mcallister, J. P. (1986) Quantitative morphology of medium-sized caudate spiny neurons in aged cats. Neurobiology of Aging 7, 277–286.
Mani, R. B., Lohr, J. B. & Jeste, D. V. (1986) Hippocampal pyramidal cells and aging in the human: a quantitative study of neuronal loss in sectors CA1 to CA4a. Experimental Neurology 94, 29–40.
Mann, D. M., Yates, P. O. & Marcyniuk, B. (1984) Changes in nerve cells of the nucleus basalis of Meynert in AlzheimerÕs disease and their relationship to ageing and to the accumulation of lipofuscin pigment. Mechanisms of Ageing & Development 25, 189–204.
Mcgeer, P. L., Mcgeer, E. G., Suzuki, J., Dolman, C. E. & Nagai, T. (1984) Aging, AlzheimerÕs disease, and the cholinergic system of the basal forebrain. Neurology 34, 741–745.
Nandy, K. (1981) Morphological changes in the cerebellar cortex of aging Macaca nemestrina. Neurobiology of Aging 2, 61–64.
Pakkenberg, B. & Gundersen, H. J. (1989) New stereological method for obtaining unbiased and efficient estimates of total nerve cell number in human brain areas. Exemplified by the mediodorsal thalamic nucleus in schizophrenics. Apmis 97, 677–681.
Pentney, R. J. (1986) Quantitative analysis of dendritic networks of Purkinje neurons during aging. Neurobiology of Aging 7, 241–248.
Pestronk, A., Drachman, D. B. & Griffin, J. W. (1980) Effects of aging on nerve sprouting and regeneration. Experimental Neurology 70, 65–82.
Peters, A., Feldman, M. L. & Vaughan, D. W. (1983) The effect of aging on the neuronal population within area 17 of adult rat cerebral cortex. Neurobiology of Aging 4, 273–282.
Peters, A., Morrison, J. H., Rosene, D. L. & Hyman, B. T. (1998) Feature article: are neurons lost from the primate cerebral cortex during normal aging? Cerebral Cortex 8, 295–300.
Pyapali, G. K. & Turner, D. A. (1996) aIncreased dendritic extent in hippocampal CA1 neurons from aged F344 rats. Neurobiology of Aging 17, 601–611.
Rogers, J., Silver, M. A., Shoemaker, W. J. & Bloom, F. E. (1980) Senescent changes in a neurobiological model system: cerebellar Purkinje cell electrophysiology and correlative anatomy. Neurobiology of Aging 1, 3–11.
Sarter, M. (1987) Animal models of brain ageing and dementia. [Review]. Comprehensive Gerontology, Section A, Clinical & Laboratory Sciences 1, 4–15.
Scheibel, M. E., Lindsay, R. D., Tomiyasu, U. & Scheibel, A. B. (1976) Progressive dendritic changes in the aginghumanlimbic system. Experimental Neurology 53, 420–430.
Sturrock, R. R. (1989a) Age related changes in Purkinje cell number in the cerebellar nodulus of the mouse. Journal für Hirnforschung 30, 757–760.
Sturrock, R. R. (1989b) Changes in neuron number in the cerebellar cortex of the ageing mouse. Journal für Hirnforschung 30, 499–503.
Sturrock, R. R. (1990) A quantitative histological study of Golgi II neurons and pale cells in different cerebellar regions of the adult and ageing mouse brain. Zeitschrift für Mikroskopisch-Anatomische Forschung 104, 705–714.
Swaab, D. F., Fliers, E. & Partiman, T. S. (1985) The suprachiasmatic nucleus of the human brain in relation to sex, age and senile dementia. Brain Resarch 342, 37–44.
Terry, R. D., Deteresa, R. & Hansen, L. A. (1987) Neocortical cell counts in normal human adult aging. Annals of Neurology 21, 530–539.
West, M. J. (1993) New stereological methods for counting neurons. Neurobiology of Aging 14, 275–285.
West, M. J., Coleman, P. D., Flood, D. G. & Troncoso, J. C. (1994) Differences in the pattern of hippocampal neuronal loss in normal ageing and AlzheimerÕs disease. Lancet 344, 769–772.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chen, S., Hillman, D.E. Dying-back of Purkinje cell dendrites with synapse loss in aging rats. J Neurocytol 28, 187–196 (1999). https://doi.org/10.1023/A:1007015721754
Issue Date:
DOI: https://doi.org/10.1023/A:1007015721754