Abstract
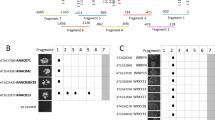
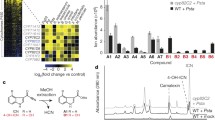
The regulation of genes in response to wounding is mediated in part by the octadecanoids 12-oxo-phytodienoic acid (OPDA), jasmonic acid (JA) and its methyl ester methyl jasmonate (MeJA). We identified, by differential display, an Arabidopsis gene (OPR3) induced after wounding. OPR3 is homologous to members of the flavin mononucleotide (FMN) binding proteins, including the old yellow enzyme (OYE) from yeast and 12-oxophytodienoate-10,11-reductase (OPR) from Arabidopsis. Transcripts of OPR3 rapidly accumulated in leaves after wounding and MeJA treatment, but they were detected in various tissues of unwounded plants at relatively low levels. Expression of the OPR3 gene was significantly reduced in wounded leaves of the coi1 mutant, indicating partial dependence on jasmonate perception for full induction of the gene. The recombinant protein of OPR3 cross-reacted with an antiserum raised against the OYE protein, and showed oxidation of β-NADPH when OPDA or 15-deoxy-Δ12,14-prostaglandin J2 (PGJ2), an analogue of OPDA, was used as substrate. β-NADPH oxidation was not observed when MeJA, which lacks the double bond in the ketone ring, was used as substrate. The recombinant OPR3 protein also showed β-NADPH oxidation activity in the presence of cyclohexenone, but not cyclohexanone, suggesting that the enzyme has specificity to cleavage of olefinic bonds in cyclic enones. The results show that the OPR3 gene product represents a new OPR of Arabidopsis induced after wounding.
Similar content being viewed by others
References
Ausubel, F.M., Brent, R., Kingston, R.E., Moore, D.D., Seidman, J.G., Smith, J.A. and Struhl, K. 1998. Current Protocols in Molecular Biology. John Wiley, New York.
Baldwin, I.T. 1998. Jasmonate-induced responses are costly but benefit plants under attack in native populations. Proc. Natl. Acad. Sci. USA 95: 8113–8118.
Bell, E. and Mullet, J.E. 1993. Characterization of an Arabidopsis lipoxygenase gene responsive to methyl jasmonate and wounding. Plant Physiol. 103: 1133–1137.
Benedetti, C.E., Costa, C.L., Turcinelli, S.R. and Arruda, P. 1998. Differential expression of a novel gene in response to coronatine, methyl jasmonate, and wounding in the coi1 mutant of Arabidopsis. Plant Physiol. 116: 1037–1042.
Bergey, D.R., Howe, G.A. and Ryan, C. 1996. Polypeptide signaling for plant defensive genes exhibits analogies to defense signaling in animals. Proc. Natl. Acad. Sci. USA 93: 12053–12058.
Biesgen, C. and Weiler, E.W. 1999. Structure and regulation of OPR1 and OPR2, two closely related genes encoding 12-oxophytodienoic acid-10,11-reductases from Arabidopsis thaliana. Planta 208: 155–165.
Brown, B.J., Deng, Z., Karplus, P.A. and Massey, V. 1998. On the active site of old yellow enzyme. Role of histidine 191 and asparagine 194. J. Biol. Chem. 273: 32753–32762.
Creelman, R.A. and Mullet, J.E. 1997. Oligosaccharins, brassinolides, and jasmonates: nontraditional regulators of plant growth, development, and gene expression. Plant Cell 9: 1211–1223.
Creelman, R.A., Tierney, M.L. and Mullet, J.E. 1992. Jasmonic acid/methyl jasmonate accumulate in wounded soybean hypocotyls and modulate wound gene expression. Proc. Natl. Acad. Sci. USA 89: 4938–4941.
Farmer, E.F. and Ryan, C.A. 1992. Octadecanoid precursors of jasmonic acid activate the synthesis of wound-inducible proteinase inhibitors. Plant Cell 4: 129–134.
Feys, B.J.F., Benedetti, C.E., Penfold, C.N. and Turner, J.G. 1994. Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male sterile, insensitive to methyl jasmonate, and resistant to a bacterial pathogen. Plant Cell 6: 751–759.
Forman, B.M., Tontonoz, P., Chen, J., Brun, R.P., Spiegelman, B.M. and Evans, R.M. 1995. 15-Deoxy-Δ12,14-prostaglandin J2 is a ligand for the adipocyte determination factor PPARγ Cell 83: 803–812.
Fox, K.M. and Karplus, P.A. 1994. Old yellow enzyme at 2 Å resolution: overall structure, ligand binding, and comparison with related flavoproteins. Structure 2: 1089–1105.
French, C.E. and Bruce, N.C. 1994. Purification and characterization of morphinone reductase from Pseudomonas putida M10. Biochem J. 301: 97–103.
French, C.E. and Bruce, N.C. 1995. Bacterial morphinone reductase is related to old yellow enzyme. Biochem. J. 312: 671–678.
Howe, G.A., Lightner, J., Browse, J. and Ryan, C.A. 1996. An octadecanoid pathway mutant (JL5) of tomato is compromised in signaling for defense against insect attack. Plant Cell 8: 2067–2077.
Iuchi, S., Yamaguchi-Shinozaki, K., Urao, T., Terao, T. and Shinozaki, K. 1996. Novel drought-inducible genes in the highly drought-tolerant cowpea: cloning of cDNAs and analysis of the expression of the corresponding genes. Plant Cell Physiol. 37: 1073–1082.
Karplus, P.A., Fox, K.M. and Massey, V. 1995. Structure-function relations for old yellow enzyme. FASEB J. 9: 1518–1526.
Kliewer, S.A., Lenhard, J.M., Wilson, T.M., Patel, I., Morris, D.C. and Lehmann, J.M. 1995. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor G and promotes adipocyte differentiation. Cell 83: 813–819.
Koch, T., Krumm, T., Jung, V., Engelberth, J. and Boland, W. 1999. Differential induction of plant volatile biosynthesis in the lima bean by early and late intermediates of the octadecanoidsignaling pathway. Plant Physiol. 121: 153–162.
Laemmli, U.K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685.
Laudert, D. and Weiler, E.W. 1998. Allene oxide synthase: a major control point in Arabidopsis thaliana octadecanoid signalling. Plant J. 15: 675–684.
Liang, P. and Pardee, A.B. 1992. Differential display of eukaryotic messenger RNA by means of polymerase chain reaction. Science 257: 967–971.
Madani, N.D., Malloy, P.J., Rodriguez-Pombo, P., Krishnan, A.V. and Feldman, D. 1994. Candida albicans estrogen-binding protein gene encodes an oxidoreductase that is inhibited by estradiol. Proc. Natl. Acad. Sci. USA 91: 922–926.
McConn, M., Creelman, R.A., Bell, E., Mullet, J.E. and Browse, J. 1997. Jasmonate is essential for insect defense in Arabidopsis. Proc. Natl. Acad. Sci. USA 94: 5473–5477.
Menke, F.L.H., Parchmann, S., Mueller, M.J., Kijne, J.W. and Memelink, J. 1999. Involvement of the octadecanoid pathway and protein phosphorylation in fungal elicitor-induced expression of terpenoid indole alkaloid biosynthetic genes in Catharanthus roseus. Plant Physiol. 119: 1289–1296.
Mueller, M.J. 1998. Radically novel prostaglandins in animals and plants: the isoprostanes. Chem. Biol. 5: 323–333.
Murashige, T. and Skoog, F. 1962. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 15: 493–497.
Niino, Y.S., Chakraborty, S., Brown, B.J. and Massey, V. 1995. A new old yellow enzyme of Saccharomyces cerevisiae. J. Biol. Chem. 270: 1983–1991.
O'Donnell, P.J., Calvert, C., Atzorn, R., Wasternack, C., Leyser, H.M.O. and Bowles, D.J. 1996. Ethylene as a signal mediating the wound response of tomato plants. Science 274: 1914–1917.
Parchmann, S. and Mueller, M.J. 1998. Evidence for the formation of dinor isoprostanes E-1 from alfa-linolenic acid in plants. J. Biol. Chem. 273: 32650–32655.
Parchmann, S., Gundlach, H. and Mueller, M.J. 1997. Induction of 12-oxo-phytodienoic acid in wounded plants and elicited plant cell cultures. Plant Physiol. 115: 1057–1064.
Penninckx, I.A.M.A., Thomma, B.P.H.J., Buchala, A., Métraux J.-P. and Broekaert, W.F. 1998. Concomitant activation of jasmonate and ethylene response pathways is required for induction of a plant defensin gene in Arabidopsis. Plant Cell 10: 2103–2113.
Rojo, E., Titarenko, E., Leó n, J., Berger, S., Vancanneyt, G. and Sánchez-Serrano, J.J. 1998. Reversible protein phosphorylation regulates jasmonic acid-dependent and-independent wound signal transduction pathways in Arabidopsis thaliana. Plant J. 13: 153–165.
Saito, K., Thiele, D.J., Davio, M., Lockridge, O. and Massey, V. 1991. The cloning and expression of a gene encoding old yellow enzyme from Saccharomyces carlsbergensis. J. Biol. Chem. 266: 20720–20724.
Sambrook, J., Fritsch, E. and Maniatis, T. 1989. Molecular Cloning: A Laboratory Manual, 2nd ed., Cold Spring Harbor Laboratory Press, Plainview, NY.
Schaller, F. and Weiler, E.W. 1997a. Molecular cloning and characterization of 12-oxophytodienoate reductase, an enzyme of the octadecanoid signaling pathway from Arabidopsis thaliana. J. Biol. Chem. 272: 28066–28072.
Schaller, F. and Weiler, E.W. 1997b. Enzymes of the octadecanoid biosynthesis in plant 12-oxo-phytodienoate 10,11-reductase. Eur. J. Biochem. 245: 294–299.
Schaller, F., Hennig, P. and Weiler, E.W. 1998. 12-Oxophytodienoate-10,11-reductase: occurrence of two isoenzymes of different specificity against stereoisomers of 12-oxophytodienoic acid. Plant Physiol. 118: 1345–1351.
Snape, J.R., Walkley, N.A., Morby, A.P., Nicklin, S. and White, G.F. 1997. Purification, properties, and sequence of glycerol trinitrate reductase from Agrobacterium radiobacter. J. Bact. 179: 7796–7802.
Staswick, P.E., Yuen, G.Y. and Lehman, C.C. 1998. Jasmonate signaling mutants of Arabidopsis are susceptible to the soil fungus Pythium irregulare. Plant J. 15: 747–754.
Stelmach, B.A., Muller, A. and Weiler, E.W. 1999. 12-oxophytodienoic acid and indole-3-acetic acid in jasmonic acidtreated tendrils of Bryonica dioica. Phytochemistry 51: 187–192.
Stott, K., Saito, K., Thiele, D.J. and Massey, V. 1993. Old yellow enzyme: the discovery of multiple isozymes and a family of related proteins. J. Biol. Chem. 268: 6097–6106.
Thomma, B.P.H.J., Eggermont, K., Penninckx, I.A.M.A., Mauch-Mani, B., Vogelsang, R., Cammue, B.P.A. and Broekaert, W.F. 1998. Separated jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc. Natl. Acad. Sci. USA 95: 15107–15111.
Titarenko, E., Rojo, E., Leó n, J. and Sánchez-Serrano, J.J. 1997. Jasmonic acid-dependent and-independent signaling pathways control wound-induced gene activation in Arabidopsis thaliana. Plant Physiol. 115: 817–826.
Vaz, A.D.N., Chakraborty, S. and Massey, V. 1995. Old yellow enzyme: aromatization of cyclic enones and the mechanism of a novel dismutation reaction. Biochemistry 34: 4246–4256.
Verwoerd, T.C., Dekker B.M.M. and Hoekema A. 1989. A smallscale procedure for the rapid isolation of plant RNAs. Nucl. Acids Res. 17: 2362.
Vick, B.A. and Zimmerman, D.C. 1986. Characterization of 12-oxophytodienenoic acid reductase in corn. Plant Physiol. 80: 202–205.
Vijayan, P., Shockey, J., Lévesque, C.A., Cook, R.J. and Browse, J. 1998. A role for jasmonate in pathogen defense of Arabidopsis. Proc. Natl. Acad. Sci. USA 95: 7209–7214.
Weber, H., Vick, B.A. and Farmer, E.E. 1997. Dinor-oxophytodienoic acid: a new hexadecanoid signal in the jasmonate family. Proc. Natl. Acad. Sci. USA 92: 10473–10478.
Xie, D.-X, Feys, B.F., James, S., Nieto-Rostro, M. and Turner, J.G. 1998. COI1: an Arabidopsis gene required for jasmonateregulated defense and fertility. Science 280: 1091–1094.
Xu, Y., Chang, P.-F.L., Liu, D., Narasimhan, M.L., Raghothama, K.G., Hasegawa, P.M. and Bressan, R.A. 1994. Plant defense genes are synergistically induced by ethylene and methyl jasmonate. Plant Cell 6: 1077–1085.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Costa, C.L., Arruda, P. & Benedetti, C.E. An Arabidopsis gene induced by wounding functionally homologous to flavoprotein oxidoreductases. Plant Mol Biol 44, 61–71 (2000). https://doi.org/10.1023/A:1006464822434
Issue Date:
DOI: https://doi.org/10.1023/A:1006464822434