Abstract
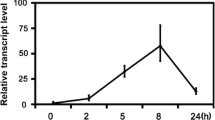
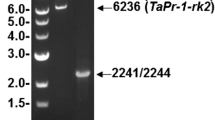
A pathogen- and salicylic acid (SA)-induced DNA-binding activity has been recently identified in tobacco that is related to a previously identified class of WRKY DNA-binding proteins. To identify members of the WRKY gene family associated with this DNA-binding activity, we have attempted to isolate those WRKY genes that are induced by pathogen infection. Using a domain-specific differential display procedure, we have isolated two tobacco WRKY genes, tWRKY3 and tWRKY4, that are rapidly induced in resistant tobacco plants after infection by tobacco mosaic virus (TMV). Both tWRK3 and tWRKY4 encode proteins with a single WRKY domain that contain the conserved WRKYGQK sequence. Unlike other isolated WRKY proteins that contain the Cys2His2 zinc motif, tWRKY3 and tWRKY4 appear to contain the Cys2HisCys zinc motif. Nonetheless, both tWRKY3 and tWRKY4 are capable of binding DNA molecules with the W-box (TTGAC) element recognized by other WRKY proteins. Expression of the tWRKY3 and tWRKY4 genes could be rapidly induced not only by TMV infection but also by SA or its biologically active analogues that are capable of inducing pathogenesis-related genes and enhanced resistance. Interestingly, induction of both genes by TMV infection was still observed in resistant tobacco plants expressing the bacterial salicylate hydroxylase gene (nahG), although the levels of induction appeared to be reduced. Identification of pathogen- and SA-induced genes encoding WRKY DNA-binding proteins should facilitate future studies on the regulation and functions of this novel group of DNA-binding proteins.
Similar content being viewed by others
References
Chen, Z., Silvan, H. and Klessig, D.F. 1991. Active oxygen species in the induction of plant systemic acquired resistance by salicylic acid. Science 262: 1883–1886.
Clarke, N.D. and Berg, J.M. 1998. Zinc fingers in Caenorhabditis elegans: finding families and probing pathways. Science 282: 2018–2022.
Conrath, U., Chen, Z., Ricigliano, J.W. and Klessig, D.F. 1995. Two inducers of plant defense responses, 2,6-dichloroisonicotinic acid and salicylic acid, inhibit catalase activity in tobacco. Proc. Natl. Acad. Sci. USA 92: 7143–7147.
de Pater, S., Greco, V., Pham, K., Memelink, J. and Kijne, J. 1996. Characterization of a zinc-dependent transcriptional activator from Arabidopsis. Nucl. Acids Res. 24: 4624–4631.
Despres, C., Subramaniam, R., Matton, D.P. and Brisson, N. 1995. The activation of the potato PR-10a gene requires the phosphorylation of the nuclear factor PBF-1. Plant Cell 7: 589–598.
Enyedi, A., Yalpani, N., Silverman, P. and Raskin, I. 1992. Localization, conjugation and function of salicylic acid in tobacco during the hypersensitive reaction to tobacco mosaic virus. Proc. Natl. Acad. Sci. USA 89: 2480–2484.
Friedrich, L., Vernooij, B., Gaffney, T., Morse, A. and Ryals, J. 1995. Characterization of tobacco plants expressing a bacterial salicylate hydroxylase gene. Plant Mol. Biol. 29: 959–968.
Fukuda, Y. 1997. Interaction of tobacco nuclear protein with an elicitor-responsive element in the promoter of a basic class I chitinase gene. Plant Mol. Biol. 34: 81–87.
Fukuda, Y. and Shinshi, H. 1994. Characterization of a novel cisacting element that is responsive to a fungal elicitor in the promoter of a tobacco class I chitinase gene. Plant Mol. Biol. 24: 485–493.
Green, P.J., Kay, S.A. and Chua, N.H. 1987. Sequence-specific interactions of a pea nuclear factor with light-responsive elements upstream of the rbcS-3A gene. EMBO J. 6: 2543–2549.
Hahn, K. and Strittmatter, G. 1994. Pathogen-defence gene prp1-1 from potato encodes an auxin-responsive glutathione Stransferase. Eur. J. Biochem. 226: 619–626
Kombrink, E. and Somssich, I.E. 1997. Pathogenesis-related proteins and plant defense. In: G. Carroll and P. Tudzynski (Eds.), TheMycota Part A, Plant Relationships, Springer-Verlag, Berlin, pp. 107–128.
Malamy, J. Hennig, J. and Klessig, D.F. 1992. Temperaturedependent induction of salicylic acid and its conjugates during the resistance response to tobacco mosaic virus infection. Plant Cell 4: 359—366.
Raventos, D., Jensen, A.B., Rask, M.B., Casacuberta, J.M., Mundy, J. and San Segundo, B. 1995. A 20 bp cis-acting element is both necessary and sufficient to mediate elicitor response of a maize PRms gene. Plant J. 7: 147–155.
Rushton, P.J. and Somssich, I.E. 1998. Transcriptional control of plant genes responsive to pathogens. Curr. Opin. Plant Biol. 1: 311–315.
Rushton, P.J., Macdonald, H., Huttly, A.K., Lazarus, C.M. and Hooley, R. 1995. Members of a new family of DNA-binding proteins bind to a conserved cis-element in the promoters of α-Amy2 genes. Plant Mol. Biol. 29: 691–702.
Rushton, P.J., Torres, J.T., Parniske, M., Wernert, P., Hahlbrock, K. and Somssich, I.E. 1996. Interaction of elicitor-induced DNAbinding proteins with elicitor response elements in the promoters of parsley PR1 genes. EMBO J. 15: 5690–500.
Sambrook, J., Fritsch, E.F. and Maniatis, T. 1989. Molecular Cloning: A Laboratory Manual, 2nd ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
Schubert, R., Fischer, R., Hain, R., Schreier, P.H., Bahnweg, G., Ernst, D. and Sandermann, H. Jr. 1997. An ozone-responsive region of the grapevine resveratrol synthase promoter differs from the basal pathogen-responsive sequence. Plant Mol. Biol. 34: 417–426.
Somssich, I.E. and Hahlbrock, K. 1998. Pathogen defense in plants: a paradigm of biological complexity. Trends Plant Sci. 3: 86–90.
van der Biezen, E.A. and Jones, J.D. 1998. Plant disease-resistance proteins and the gene-for-gene concept. Trends Biochem.. Sci. 23: 454–456.
Wang, Z., Yang, P., Fan, B. and Chen, Z. 1998. An oligo selection procedure for identification of sequence-specific DNA-binding activities associated with the plant defense response. Plant J. 16: 515–522.
Warner, S.A., Scott, R. and Draper, J. 1993. Isolation of an asparagus intracellular PR gene (AoPR1) wound-responsive promoter by the inverse polymerase chain reaction and its characterization in transgenic tobacco. Plant J. 3: 191–201.
Warner, S.A., Gill, A. and Draper, J. 1994. The developmental expression of the asparagus intracellular PR protein (AoPR1) gene correlates with sites of phenylpropanoid biosynthesis. Plant J. 6: 31–43.
Whitham, S., McCormick, S. and Baker, B. 1996. The N gene of tobacco confers resistance to tobacco mosaic virus in transgenic tomato. Proc. Natl. Acad. Sci. USA 93: 8776–8781.
Yang, Y., Shah, J. and Klessig, D.F. 1997. Signal perception and transduction in plant defense responses. Genes Dev. 11: 1621–1639.
Yang, P., Wang, Z., Fan, B., Chen, C. and Chen, Z. 1999. A pathogen-and salicylic acid-induced WRKY DNA-binding activity recognizes the elicitor response element of the tobacco class I chitinase gene promoter. Plant J. 18: 141–149.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chen, C., Chen, Z. Isolation and characterization of two pathogen- and salicylic acid-induced genes encoding WRKY DNA-binding proteins from tobacco. Plant Mol Biol 42, 387–396 (2000). https://doi.org/10.1023/A:1006399311615
Issue Date:
DOI: https://doi.org/10.1023/A:1006399311615