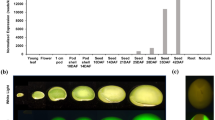
Abstract
The pigmented seed coats of several soybean (Glycine max (L.) Merr.) plant introductions and isolines have unusual defects that result in cracking of the mature seed coat exposing the endosperm and cotyledons. It has previously been shown that the T (tawny) locus that controls the color of trichomes on stems and leaves also has an effect on both the structure and pigmentation of the seed coat. Distribution of pigmentation on the seed coat is controlled by alleles of the I (inhibitor) locus. It was also found that total seed coat proteins were difficult to extract from pigmented seed coats with i T genotypes because they have procyanidins that exhibit tannin properties. We report that the inclusion of poly-L-proline in the extraction buffer out-competes proteins for binding to procyanidins. Once this problem was solved, we examined expression of the proline-rich cell wall proteins PRP1 and PRP2 in pigmented genotypes with the dominant T allele. We found that both homozygous i T and i t genotypes have reduced soluble PRP1 levels. The epistatic interaction of the double recessive genotype at both loci is necessary to produce the pigmented, defective seed coat phenotype characteristic of seed coats with the double recessive i and t alleles. This implies a novel effect of an enzyme in the flavonoid pathway on seed coat structure in addition to its effect on flavonoids, anthocyanidins, and proanthocyanidins. No soluble PRP1 polypeptides were detectable in pigmented seed coats (i T genotypes) of isolines that also display a net-like pattern of seed coat cracking, known as the Net defect. PRP2 was also absent in one of the these lines. However, both PRP1 and PRP2 cytoplasmic mRNAs were found in the Net-defective seed coats. Together with in vitro translation studies, these results suggest that the absence of soluble PRP polypeptides in the defective Net lines is post-translational and could be due to a more rapid or premature insolubilization of PRP polypeptides within the cell wall matrix.
Similar content being viewed by others
References
Bradley, D.J., Kjellbom, P. and Lamb, C.J. 1992. Elicitor-and wound-induced oxidative cross-linking of a proline-rich plant cell wall protein: a novel, rapid defense response. Cell 70: 21-30.
Brady, J.D., Sadler, I.H. and Fry, S.C. 1996. Di-isodityrosine, a novel tetrameric derivative of tyrosine in plant cell wall proteins: a new potential cross-link. Biochem. J. 315: 323-327.
Bradford, M.M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248-254.
Brisson, L.F., Tenhaken, R. and Lamb, C. 1994. Function of oxidative cross-linking of cell wall structural proteins in plant disease resistance. Plant Cell 6: 1703-1712.
Hong, J.C., Nagao, R.T. and Key, J.L. 1987. Characterization and sequence analysis of a developmentally regulated putative cell wall protein gene isolated from soybean. J. Biol. Chem. 262: 8367-8376.
Hong, J.C., Nagao, R.T. and Key, J.L. 1989. Developmentally regulated expression of soybean proline-rich cell wall protein genes. Plant Cell 1: 937-943.
Hong, J.C., Cheong, Y.H., Nagao, R.T., Bahk, J.D., Cho, M.J. and Key, J.L. 1994. Isolation and characterization of three soybean extensin cDNAs. Plant Physiol. 104: 793-796.
Lindstrom, J.T. and Vodkin, L.O. 1991. A soybean cell wall protein is affected by seed color genotype. Plant Cell 3: 561-571.
Nicholas, C.D., Lindstrom, J.T. and Vodkin, L.O. 1993. Variation of proline-rich cell wall proteins in soybean lines with anthocyanin mutations. Plant Mol. Biol. 21: 145-156.
Palmer, R.G. and Kilen, T.C. 1987. Qualitative genetics and cytogenetics. In: J.R. Wilcox (Ed.), Soybeans: Improvement, Production, and Uses. American Society of Agronomy Publishers, Madison, WI, pp. 135-197.
Scalbert, A. 1991. Antimicrobial patterns of tannins. Phytochemistry 30: 3875-3883.
Showalter, A.M. 1993. Structure and function of plant cell wall proteins. Plant Cell 5: 9-23.
Showalter, A.M., Bell, J.N., Cramer, C.L., Bailey, J.A., Varner, J.E. and Lamb, C.J. 1985. Accumulation of hydroxyproline-rich glycoprotein mRNAs in response to fungal elicitor and infection. Proc. Natl. Acad. Sci. USA 82: 6551-6555.
Stafford, H.A. 1990. Pathway to proanthocyanidins (condensed tannins), flavan-3-ols, and unsubstituted flavans. In: Flavonoid Metabolism. CRC Press, Boca Raton, FL, p. 66.
Todd, J.J. and Vodkin, L.O. 1993. Pigmented soybean (Glycine max) seed coats accumulate proanthocyanidins during development. Plant Physiol. 102: 663-670.
Todd, J.J. and Vodkin, L.O. 1996. Duplications that suppress and deletions that restore expression from a chalcone synthase multigene family. Plant Cell 8: 687-699.
Vodkin, L.O. and Raikhel, N.V. 1986. Soybean lectin and related proteins in seeds and roots of LeC and Le_ soybean varieties. Plant Physiol. 81: 558-565.
Wang, C.S. and Vodkin, L.O. 1994. Extraction of RNA from tissues containing high levels of procyanidins that bind RNA. PlantMol. Biol. Rep. 12: 132-145.
Wang, C.S., Todd, J.J. and Vodkin, L.O. 1994. Chalcone synthase mRNA and activity are reduced in yellow soybean seed coats with dominant I alleles. Plant Physiol. 105: 739-748.
Wojtaszek, P., Trethowan, J. and Bolwell, G.P. 1995. Specificity in the immobilisation of cell wall proteins in response to different elicitor molecules in suspension-cultured cells of French bean (Phaseolus vulgaris L.). Plant Mol. Biol. 28: 1075-1087.
Wyatt, R.E., Nagao, R.T. and Key, J.L. 1992. Patterns of soybean proline-rich protein gene expression. Plant Cell 4: 99-110.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Percy, J.D., Philip, R. & Vodkin, L.O. A defective seed coat pattern (Net) is correlated with the post-transcriptional abundance of soluble proline-rich cell wall proteins. Plant Mol Biol 40, 603–613 (1999). https://doi.org/10.1023/A:1006221115522
Issue Date:
DOI: https://doi.org/10.1023/A:1006221115522