Summary
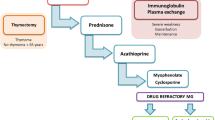
Myasthenia gravis (MG) is a prototypic antibody-mediated neurological autoimmune disorder. Herein we characterize modern treatment algorithms that are adapted to disease severity, and introduce the current principles of escalating strategies for MG treatment. In non-thymoma patients younger than about 50 years of age and with generalized weakness, a complete early (but not urgent) thymectomy is considered as state-of-the-art on the basis of circumstantial evidence and expert opinion. In up to 10% of patients, MG is associated with a thymoma (i.e., is of paraneoplastic origin). The best surgical type of procedure is still under debate.
Myasthenic crisis is best treated by plasmapheresis, mostly combined with immunoabsorption techniques. Intravenous immunoglobulins are a reasonable alternative, but a shortage in supplies and high prices limit their use. In generalized MG, a wide array of immunosuppressive treatments has been established, although not formally tested in double-blind, prospective trials. With regard to immunosuppression, azathioprine is still the standard baseline treatment, often combined with initial corticosteroids. In rare patients with an inborn hepatic enzyme deficiency of thiomethylation, azathioprine may be substituted by mycophenolate mofetil. Severe cases may benefit from combined immunosuppression with corticosteroids, cyclosporine A, and even with moderate doses of methotrexate or cyclophosphamide. Tacrolimus is under investigation.
In refractory cases, immunoablation via high-dose cyclophosphamide followed by trophic factors such as granulocyte colony-stimulating factor has also been suggested. In the future we may face an increased use of novel, B-cell, or T-cell-directed monoclonal antibodies.
Similar content being viewed by others
References
Drachman DB. Myasthenia gravis. N Engl J Med 1994;330: 1797–1810.
Hohlfeld R, Wekerle H. The immunopathogenesis of myasthenia gravis. In: Engel AG, editor. Myasthenia gravis and myasthenic syndromes. Oxford: Oxford University Press;1999. pp. 87–110.
Vincent A, Bowen J, Newsom-Davis J, McConville J. Seronegative generalised myasthenia gravis: clinical features, antibodies and their targets. Lancet Neurol 2003;2: 99–106.
Hohlfeld R, Melms A, Schneider C, Toyka KV, Drachman DB. Therapy of myasthenia gravis and myasthenic syndromes In: Brandt T, Caplan LR, Dichgans J, Diener HC, Kennard C, editors. Neurological disorders: course and treatment. Philadelphia: Elsevier; 2003. pp. 1341–1362.
Wekerle H, Ketelsen UP. Intrathymic pathogenesis and dual genetic control of myasthenia gravis. Lancet 1977;1: 678–680.
Kirchner T, Schalke B, Melms A, von Kugelgen T, Muller-Hermelink HK. Immunohistological patterns of non-neoplastic changes in the thymus in Myasthenia gravis. Virchows Arch B Cell Pathol Incl Mol Pathol 1986;52: 237–257.
Farrugia ME, Robson MD, Clover L, et al. MRI and clinical studies of facial and bulbar muscle involvement in MuSK antibody-associated myasthenia gravis. Brain 2006;129: 1481–1492.
Leite MI, Strobel P, Jones M, et al. Fewer thymic changes in MuSK antibody-positive than in MuSK antibody-negative MG. Ann Neurol 2005;57: 444–448.
Strobel P, Bauer A, Puppe B, et al. Tumor recurrence and survival in patients treated for thymomas and thymic squamous cell carcinomas: a retrospective analysis. J Clin Oncol 2004;22: 1501–1509.
Schneider-Gold C, Toyka KV. Myasthenia gravis: pathogenesis and immunotherapy (English). Dtsch Ä rzteblatt 2007;104: A420-A426.
Gold R, Dalakas MC, Toyka KV. Immunotherapy in autoimmune neuromuscular disorders. Lancet Neurol 2003;2: 22–32.
Muller-Hermelink HK, Marx A. Thymoma. Curr Opin Oncol 2000;12: 426–433.
Schneider-Gold C, Gajdos P, Toyka KV, Hohlfeld RR. Corticosteroids for myasthenia gravis. Cochrane Database Syst Rev 2005;CD002828.
Gronseth GS, Barohn RJ. Practice parameter: thymectomy for autoimmune myasthenia gravis (an evidence-based review)-Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2000;55: 7–15.
Mertens HG, Hertel G, Reuther P, Ricker K. Effect of immunosuppressive drugs (azathioprine). Ann N Y Acad Sci 1981;377: 691–699.
Palace J, Newsom-Davis J, Lecky B; Myasthenia Gravis Study Group. A randomized double-blind trial of prednisolone alone or with azathioprine in myasthenia gravis. Neurology 1998;50: 1778–1783.
Gold R, Toyka KV. Immuntherapie neurologischer Erkrankungen. UniMed Verlag; Bremen: 2006.
Hohlfeld R, Toyka KV, Besinger UA, Gerhold B, Heininger K. Myasthenia gravis: reactivation of clinical disease and of autoimmune factors after discontinuation of long-term azathioprine. Ann Neurol 1985;17: 238–242.
Schneider-Gold C, Hartung HP, Gold R. Mycophenolate mofetil and tacrolimus: new therapeutic options in neuroimmunological diseases. Muscle & Nerve 2006;34: 284–291.
Benatar M, Rowland LP. The muddle of mycophenolate mofetil in myasthenia. Neurology 2008;71: 390–391.
Schneider C, Gold R, Reiners K, Toyka KV. Mycophenolate mofetil in the therapy of severe myasthenia gravis. Eur Neurol 2001;46: 79–82.
Sanders DB, Hart IK, Mantegazza R, et al. An international, phase III, randomized trial of mycophenolate mofetil in myasthenia gravis. Neurology 2008;71: 400–406.
Gold R, Stangel M, Dalakas MC. Drug insight: the use of intravenous immunoglobulin in neurology-therapeutic considerations and practical issues. Nat Clin Pract Neurol 2007;3: 36–44.
Gajdos P, Chevret S, Clair B, Tranchant C, Chastang C. Clinical trial of plasma exchange and high-dose intravenous immunoglobulin in myasthenia gravis. Ann Neurol 1997;41: 789–796.
Gajdos P, Tranchant C, Clair B, et al. Treatment of myasthenia gravis exacerbation with intravenous immunoglobulin-a randomized double-blind clinical trial. Arch Neurol 2005;62: 1689–1693.
Zinman L, Ng E, Bril V. IV immunoglobulin in patients with myasthenia gravis-a randomized controlled trial. Neurology 2007;68: 837–841.
Lehmann HC, Hartung HP, Hetzel GR, Stuve O, Kieseier BC. Plasma exchange in neuroimmunological disorders: part 2. Treatment of neuromuscular disorders. Arch Neurol 2006;63: 1066–1071.
Bufler J, Kahlert S, Tzartos S, Toyka KV, Maelicke A, Franke C. Activation and blockade of mouse muscle nicotinic channels by antibodies directed against the binding site of the acetylcholine receptor. J Physiol (Lond) 1996;492: 107–114.
Heininger K, Hartung H-P, Toyka KV, Gaczkowski A, Borberg H. Therapeutic plasma exchange in myasthenia gravis: semiselective adsorption of Anti-AChR autoantibodies with tryptophane-linked polyvinylalcohol gels. Ann N Y Acad Sci 1987;505: 898–900.
Flachenecker P, Taleghani BM, Gold R, Grossmann R, Wiebecke D, Toyka KV. Treatment of severe myasthenia gravis with protein A immunoadsorption and cyclophosphamide. Transfus Sci 1998;19(Suppl): 43–46.
Hauser SL, Waubant E, Arnold DL, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med 2008;358: 676–688.
Martin F, Chan AC. B cell immunobiology in disease: evolving concepts from the clinic. Annu Rev Immunol 2006;24: 467–496.
Chan A, Lee DH, Linker R, Mohr A, Toyka KV, Gold R. Rescue therapy with anti-CD20 treatment in neuroimmunologic break-through disease. J Neurol 2007;254: 1604–1606.
Thakre M, Inshasi J, Marashi M. Rituximab in refractory MuSK antibody myasthenia gravis. J Neurol 2007;254: 968–969.
Hain B, Jordan K, Deschauer M, Zierz S. Successful treatment of musk antibody-positive myasthenia gravis with rituximab. Muscle & Nerve 2006;33: 575–580.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gold, R., Schneider-Gold, C. Current and future standards in treatment of myasthenia gravis. Neurotherapeutics 5, 535–541 (2008). https://doi.org/10.1016/j.nurt.2008.08.011
Published:
Issue Date:
DOI: https://doi.org/10.1016/j.nurt.2008.08.011