Abstract
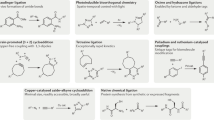
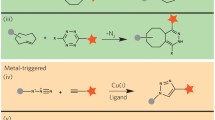
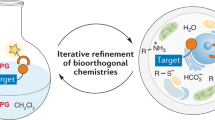
Bioorthogonal chemistry has emerged as a new powerful tool that facilitates the study of structure and function of biomolecules in their native environment. A wide variety of bioorthogonal reactions that can proceed selectively and efficiently under physiologically relevant conditions are now available. The common features of these chemical reactions include: fast kinetics, tolerance to aqueous environment, high selectivity and compatibility with naturally occurring functional groups. The design and development of new chemical transformations in this direction is an important step to meet the growing demands of chemical biology. This chapter aims to introduce the reader to the field by providing an overview on general principles and strategies used in bioorthogonal chemistry. Special emphasis is given to cycloaddition reactions, namely to 1,3-dipolar cycloadditions and Diels–Alder reactions, as chemical transformations that play a predominant role in modern bioconjugation chemistry. The recent advances have established these reactions as an invaluable tool in modern bioorthogonal chemistry. The key aspects of the methodology as well as future outlooks in the field are discussed.
















Similar content being viewed by others
Change history
25 February 2016
An erratum to this article has been published.
References
Kolb HC, Finn MG, Sharpless KB (2001) Click chemistry: diverse chemical function from a few good reactions. Angew Chem Int Ed 40(11):2004–2021. doi:10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5
Dedecker P, De Schryver FC, Hofkens J (2013) Fluorescent proteins: shine on, you crazy diamond. J Am Chem Soc 135(7):2387–2402. doi:10.1021/ja309768d
Nienhaus K, Nienhaus GU (2014) Fluorescent proteins for live-cell imaging with super-resolution. Chem Soc Rev 43(4):1088–1106. doi:10.1039/c3cs60171d
Hang HC, Yu C, Kato DL, Bertozzi CR (2003) A metabolic labeling approach toward proteomic analysis of mucin-type O-linked glycosylation. P Natl Acad Sci USA 100(25):14846–14851. doi:10.1073/pnas.2335201100
Rideout D, Calogeropoulou T, Jaworski J, McCarthy M (1990) Synergism through direct covalent bonding between agents: a strategy for rational design of chemotherapeutic combinations. Biopolymers 29(1):247–262. doi:10.1002/bip.360290129
Griffin BA, Adams SR, Tsien RY (1998) Specific covalent labeling of recombinant protein molecules inside live cells. Science 281(5374):269–272. doi:10.1126/science.281.5374.269
Prescher JA, Bertozzi CR (2005) Chemistry in living systems. Nat Chem Biol 1(1):13–21. doi:10.1038/nchembio0605-13
Sletten EM, Bertozzi CR (2009) Bioorthogonal chemistry: fishing for selectivity in a sea of functionality. Angew Chem Int Ed 48(38):6974–6998. doi:10.1002/anie.200900942
Merkel M, Peewasan K, Arndt S, Ploschik D, Wagenknecht HA (2015) Copper-free postsynthetic labeling of nucleic acids by means of bioorthogonal reactions. ChemBioChem 16(11):1541–1553. doi:10.1002/cbic.201500199
Tang W, Becker ML (2014) “Click” reactions: a versatile toolbox for the synthesis of peptide-conjugates. Chem Soc Rev 43(20):7013–7039. doi:10.1039/c4cs00139g
Hocek M (2014) Synthesis of base-modified 2’-deoxyribonucleoside triphosphates and their use in enzymatic synthesis of modified DNA for applications in bioanalysis and chemical biology. J Org Chem 79(21):9914–9921. doi:10.1021/jo5020799
Davis L, Chin JW (2012) Designer proteins: applications of genetic code expansion in cell biology. Nat Rev Mol Cell Bio 13(3):168–182. doi:10.1038/nrm3286
Dumas A, Lercher L, Spicer CD, Davis BG (2015) Designing logical codon reassignment—expanding the chemistry in biology. Chem Sci 6(1):50–69. doi:10.1039/c4sc01534g
Du J, Meledeo MA, Wang ZY, Khanna HS, Paruchuri VDP, Yarema KJ (2009) Metabolic glycoengineering: sialic acid and beyond. Glycobiology 19(12):1382–1401. doi:10.1093/glycob/cwp115
Jao CY, Roth M, Welti R, Salic A (2009) Metabolic labeling and direct imaging of choline phospholipids in vivo. P Natl Acad Sci USA 106(36):15332–15337. doi:10.1073/pnas.0907864106
Rieder U, Luedtke NW (2014) Alkene-Tetrazine ligation for imaging cellular DNA. Angew Chem Int Ed 53(35):9168–9172. doi:10.1002/anie.201403580
Shieh P, Siegrist MS, Cullen AJ, Bertozzi CR (2014) Imaging bacterial peptidoglycan with near-infrared fluorogenic azide probes. P Natl Acad Sci USA 111(15):5456–5461. doi:10.1073/pnas.1322727111
Huisgen R (1963) 1,3-dipolar cycloadditions. past and future. Angew Chem Int Ed 2(10):565–598. doi:10.1002/anie.196305651
Najera C, Sansano JM (2009) 1,3-Dipolar cycloadditions: applications to the synthesis of antiviral agents. Org Biomol Chem 7(22):4567–4581. doi:10.1039/b913066g
Padwa A, Pearson WH (2002) Synthetic applications of 1,3-dipolar cycloaddition chemistry toward heterocycles and natural products. Chem Heterocylcl Comp 59: John Wiley & Sons, Inc
Pearson WH (2002) Alkaloid synthesis via [3 + 2] cycloadditions. Pure Appl Chem 74(8):1339–1347. doi:10.1351/pac200274081339
Ess DH, Houk KN (2008) Theory of 1,3-dipolar cycloadditions: distortion/interaction and frontier molecular orbital models. J Am Chem Soc 130(31):10187–10198. doi:10.1021/ja800009z
Butler RN, Cunningham WJ, Coyne AG, Burke LA (2004) The influence of water on the rates of 1,3-dipolar cycloaddition reactions: trigger points for exponential rate increases in water-organic solvent mixtures. Water-super versus water-normal dipolarophiles. J Am Chem Soc 126(38):11923–11929. doi:10.1021/ja040119y
Michael A (1893) Ueber die Einwirkung von Diazobenzolimid auf Acetylendicarbonsäuremethylester. J Prakt Chem 48:94–95. doi:10.1002/prac.18930480114
Rostovtsev VV, Green LG, Fokin VV, Sharpless KB (2002) A stepwise Huisgen cycloaddition process: copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew Chem Int Ed 41(14):2596–2599. doi:10.1002/1521-3773(20020715)41:14<2596::Aid-Anie2596>3.0.Co;2-4
Tornoe CW, Christensen C, Meldal M (2002) Peptidotriazoles on solid phase: [1-3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J Org Chem 67(9):3057–3064. doi:10.1021/jo011148j
Worrell BT, Malik JA, Fokin VV (2013) Direct evidence of a dinuclear copper intermediate in Cu(I)-catalyzed azide-alkyne cycloadditions. Science 340(6131):457–460. doi:10.1126/science.1229506
Lahann J (2009) Click chemistry for biotechnology and materials science. Wiley, Hoboken. doi:10.1002/9780470748862
El-Sagheer AH, Brown T (2010) Click chemistry with DNA. Chem Soc Rev 39(4):1388–1405. doi:10.1039/b901971p
Lallana E, Riguera R, Fernandez-Megia E (2011) Reliable and efficient procedures for the conjugation of biomolecules through huisgen azide-alkyne cycloadditions. Angew Chem Int Ed 50(38):8794–8804. doi:10.1002/anie.201101019
Hanni KD, Leigh DA (2010) The application of CuAAC ‘click’ chemistry to catenane and rotaxane synthesis. Chem Soc Rev 39(4):1240–1251. doi:10.1039/b901974j
Ganesh V, Sudhir VS, Kundu T, Chandrasekaran S (2011) 10 years of click chemistry: synthesis and applications of ferrocene-derived triazoles. Chem-Asian J 6(10):2670–2694. doi:10.1002/asia.201100408
Johnson RP, John JV, Kim I (2013) Recent developments in polymer-block-polypeptide and protein-polymer bioconjugate hybrid materials. Eur Polym J 49(10):2925–2948. doi:10.1016/j.eurpolymj.2013.04.017
Tron GC, Pirali T, Billington RA, Canonico PL, Sorba G, Genazzani AA (2008) Click chemistry reactions in medicinal chemistry: applications of the 1,3-dipolar cycloaddition between azides and alkynes. Med Res Rev 28(2):278–308. doi:10.1002/med.20107
Thirumurugan P, Matosiuk D, Jozwiak K (2013) Click chemistry for drug development and diverse chemical-biology applications. Chem Rev 113(7):4905–4979. doi:10.1021/cr200409f
Kennedy DC, McKay CS, Legault MCB, Danielson DC, Blake JA, Pegoraro AF, Stolow A, Mester Z, Pezacki JP (2011) Cellular consequences of copper complexes used to catalyze bioorthogonal click reactions. J Am Chem Soc 133(44):17993–18001. doi:10.1021/ja2083027
Besanceney-Webler C, Jiang H, Zheng TQ, Feng L, del Amo DS, Wang W, Klivansky LM, Marlow FL, Liu Y, Wu P (2011) Increasing the efficacy of bioorthogonal click reactions for bioconjugation: a comparative study. Angew Chem Int Ed 50(35):8051–8056. doi:10.1002/anie.201101817
Wang W, Hong SL, Tran A, Jiang H, Triano R, Liu Y, Chen X, Wu P (2011) Sulfated ligands for the copper(I)-catalyzed azide-alkyne cycloaddition. Chem-Asian J 6(10):2796–2802. doi:10.1002/asia.201100385
Agard NJ, Prescher JA, Bertozzi CR (2004) A strain-promoted [3 + 2] azide-alkyne cycloaddition for covalent modification of blomolecules in living systems. J Am Chem Soc 126(46):15046–15047. doi:10.1021/ja0449981
Baskin JM, Prescher JA, Laughlin ST, Agard NJ, Chang PV, Miller IA, Lo A, Codelli JA, Bertozzi CR (2007) Copper-free click chemistry for dynamic in vivo imaging. P Natl Acad Sci USA 104(43):16793–16797. doi:10.1073/pnas.0707090104
Laughlin ST, Bertozzi CR (2009) In vivo imaging of caenorhabditis elegans Glycans. ACS Chem Biol 4(12):1068–1072. doi:10.1021/cb900254y
Gutsmiedl K, Wirges CT, Ehmke V, Carell T (2009) Copper-free “click” modification of DNA via nitrile oxide-norbornene 1,3-dipolar cycloaddition. Org Lett 11(11):2405–2408. doi:10.1021/ol9005322
Jawalekar AM, Reubsaet E, Rutjes FPJT, van Delft FL (2011) Synthesis of isoxazoles by hypervalent iodine-induced cycloaddition of nitrile oxides to alkynes. Chem Commun 47(11):3198–3200. doi:10.1039/c0cc04646a
Sanders BC, Friscourt F, Ledin PA, Mbua NE, Arumugam S, Guo J, Boltje TJ, Popik VV, Boons GJ (2011) Metal-free sequential [3 + 2]-dipolar cycloadditions using cyclooctynes and 1,3-dipoles of different reactivity. J Am Chem Soc 133(4):949–957. doi:10.1021/ja1081519
Mckay CS, Moran J, Pezacki JP (2010) Nitrones as dipoles for rapid strain-promoted 1,3-dipolar cycloadditions with cyclooctynes. Chem Commun 46(6):931–933. doi:10.1039/b921630h
McKay CS, Chigrinova M, Blake JA, Pezacki JP (2012) Kinetics studies of rapid strain-promoted [3 + 2]-cycloadditions of nitrones with biaryl-aza-cyclooctynone. Org Biomol Chem 10(15):3066–3070. doi:10.1039/c2ob07165g
MacKenzie DA, Pezacki JP (2014) Kinetics studies of rapid strain-promoted [3 + 2] cycloadditions of nitrones with bicyclo[6.1.0]nonyne. Can J Chem 92(4):337–340. doi:10.1139/cjc-2013-0577
McKay CS, Blake JA, Cheng J, Danielson DC, Pezacki JP (2011) Strain-promoted cycloadditions of cyclic nitrones with cyclooctynes for labeling human cancer cells. Chem Commun 47(36):10040–10042. doi:10.1039/c1cc13808a
Ning XH, Temming RP, Dommerholt J, Guo J, Ania DB, Debets MF, Wolfert MA, Boons GJ, van Delft FL (2010) Protein modification by strain-promoted alkyne-nitrone cycloaddition. Angew Chem Int Ed 49(17):3065–3068. doi:10.1002/anie.201000408
Temming RP, Eggermont L, van Eldijk MB, van Hest JCM, van Delft FL (2013) N-terminal dual protein functionalization by strain-promoted alkyne-nitrone cycloaddition. Org Biomol Chem 11(17):2772–2779. doi:10.1039/c3ob00043e
Wang XS, Lee YJ, Liu WSR (2014) The nitrilimine-alkene cycloaddition is an ultra rapid click reaction. Chem Commun 50(24):3176–3179. doi:10.1039/c3cc48682f
Wang YZ, Vera CIR, Lin Q (2007) Convenient synthesis of highly functionalized pyrazolines via mild, photoactivated 1,3-dipolar cycloaddition. Org Lett 9(21):4155–4158. doi:10.1021/ol7017328
Song W, Wang Y, Qu J, Madden MM, Lin Q (2008) A photoinducible 1,3-dipolar cycloaddition reaction for rapid, selective modification of tetrazole-containing proteins. Angew Chem Int Ed 47(15):2832–2835. doi:10.1002/anie.200705805
Kaya E, Vrabel M, Deiml C, Prill S, Fluxa VS, Carell T (2012) A genetically encoded norbornene amino acid for the mild and selective modification of proteins in a copper-free click reaction. Angew Chem Int Ed 51(18):4466–4469. doi:10.1002/anie.201109252
Ramil CP, Lin Q (2014) Photoclick chemistry: a fluorogenic light-triggered in vivo ligation reaction. Curr Opin Chem Biol 21:89–95. doi:10.1016/j.cbpa.2014.05.024
Lim RKV, Lin Q (2011) Photoinducible bioorthogonal chemistry: a spatiotemporally controllable tool to visualize and perturb proteins in live cells. Acc Chem Res 44(9):828–839. doi:10.1021/ar200021p
Padwa A, Smolanof J (1971) Photocycloaddition of arylazirenes with electron-deficient olefins. J Am Chem Soc 93(2):548–550. doi:10.1021/ja00731a056
Lim RKV, Lin Q (2010) Azirine ligation: fast and selective protein conjugation via photoinduced azirine-alkene cycloaddition. Chem Commun 46(42):7993–7995. doi:10.1039/c0cc02863k
Huisgen R, Gotthardt H, Grashey R (1962) Reactions of sydnones with alkenes. Angew Chem Int Ed 1(1):49. doi:10.1002/anie.196200491
Huisgen R, Grashey R, Gotthardt H, Schmidt R (1962) 1,3-dipolar additions of sydnones to alkynes. a new route into the pyrazole series. Angew Chem Int Ed 1(1):48–49. doi:10.1002/anie.196200482
Kolodych S, Rasolofonjatovo E, Chaumontet M, Nevers MC, Creminon C, Taran F (2013) Discovery of chemoselective and biocompatible reactions using a high-throughput immunoassay screening. Angew Chem Int Ed 52(46):12056–12060. doi:10.1002/anie.201305645
Wallace S, Chin JW (2014) Strain-promoted sydnone bicyclo-[6.1.0]-nonyne cycloaddition. Chem Sci 5(5):1742–1744. doi:10.1039/c3sc53332h
Plougastel L, Koniev O, Specklin S, Decuypere E, Creminon C, Buisson DA, Wagner A, Kolodych S, Taran F (2014) 4-Halogeno-sydnones for fast strain promoted cycloaddition with bicyclo-[6.1.0]-nonyne. Chem Commun 50(66):9376–9378. doi:10.1039/c4cc03816a
Myers EL, Raines RT (2009) A phosphine-mediated conversion of azides into diazo compounds. Angew Chem Int Ed 48(13):2359–2363. doi:10.1002/anie.200804689
McGrath NA, Raines RT (2012) Diazo compounds as highly tunable reactants in 1,3-dipolar cycloaddition reactions with cycloalkynes. Chem Sci 3(11):3237–3240. doi:10.1039/c2sc20806g
Andersen KA, Aronoff MR, McGrath NA, Raines RT (2015) Diazo groups endure metabolism and enable chemoselectivity in cellulo. J Am Chem Soc 137(7):2412–2415. doi:10.1021/ja5095815
Friscourt F, Fahrni CJ, Boons G-J (2015) Fluorogenic strain-promoted alkyne-diazo cycloadditions. Chem A Eur J 21(40):13996–14001. doi:10.1002/chem.201502242
Diels O, Alder K (1928) Synthesen in der hydroaromatischen Reihe. Justus Liebigs Annalen der Chemie 460(1):98–122. doi:10.1002/jlac.19284600106
Nicolaou KC, Snyder SA, Montagnon T, Vassilikogiannakis G (2002) The Diels-Alder reaction in total synthesis. Angew Chem Int Ed 41(10):1668–1698. doi:10.1002/1521-3773(20020517)41:10<1668::Aid-Anie1668>3.0.Co;2-Z
Rideout DC, Breslow R (1980) Hydrophobic acceleration of diels-alder reactions. J Am Chem Soc 102(26):7816–7817. doi:10.1021/ja00546a048
Otto S, Blokzijl W, Engberts JBFN (1994) Diels-alder reactions in water—effects of hydrophobicity and hydrogen-bonding. J Org Chem 59(18):5372–5376. doi:10.1021/jo00097a045
Chalker JM, Bernardes GJL, Lin YA, Davis BG (2009) Chemical modification of proteins at cysteine: opportunities in chemistry and biology. Chem-Asian J 4(5):630–640. doi:10.1002/asia.200800427
Seelig B, Jaschke A (1997) Site-specific modification of enzymatically synthesized RNA: transcription initiation and Diels-Alder reaction. Tetrahedron Lett 38(44):7729–7732. doi:10.1016/S0040-4039(97)10151-4
Hill KW, Taunton-Rigby J, Carter JD, Kropp E, Vagle K, Pieken W, McGee DPC, Husar GM, Leuck M, Anziano DJ, Sebesta DP (2001) Diels-Alder bioconjugation of diene-modified oligonucleotides. J Org Chem 66(16):5352–5358. doi:10.1021/jo0100190
de Araujo AD, Palomo JM, Cramer J, Kohn M, Schroder H, Wacker R, Niemeyer C, Alexandrov K, Waldmann H (2006) Diels-Alder ligation and surface immobilization of proteins. Angew Chem Int Ed 45(2):296–301. doi:10.1002/anie.200502266
Marchan V, Ortega S, Pulido D, Pedroso E, Grandas A (2006) Diels-Alder cycloadditions in water for the straightforward preparation of peptide-oligonucleotide conjugates. Nucleic Acids Res 34(3):e24. doi:10.1093/nar/gnj020
Li Q, Dong T, Liu X, Lei X (2013) A bioorthogonal ligation enabled by click cycloaddition of o-Quinolinone Quinone methide and vinyl thioether. J Am Chem Soc 135(13):4996–4999. doi:10.1021/ja401989p
Carboni RA, Lindsey RV (1959) Reactions of tetrazines with unsaturated compounds. A new synthesis of pyridazines. J Am Chem Soc 81(16):4342–4346. doi:10.1021/ja01525a060
Boger DL (1986) Diels-Alder reactions of heterocyclic aza dienes, Scope and applications. Chem Rev 86(5):781–793. doi:10.1021/cr00075a004
Blackman ML, Royzen M, Fox JM (2008) Tetrazine ligation: fast bioconjugation based on inverse-electron-demand Diels-Alder reactivity. J Am Chem Soc 130(41):13518–13519. doi:10.1021/ja8053805
Devaraj NK, Weissleder R, Hilderbrand SA (2008) Tetrazine-based cycloadditions: application to pretargeted live cell imaging. Bioconjugate Chem 19(12):2297–2299. doi:10.1021/bc8004446
Chen WX, Wang DZ, Dai CF, Hamelberg D, Wang BH (2012) Clicking 1,2,4,5-tetrazine and cyclooctynes with tunable reaction rates. Chem Commun 48(12):1736–1738. doi:10.1039/c2cc16716f
Patterson DM, Nazarova LA, Xie B, Kamber DN, Prescher JA (2012) Functionalized cyclopropenes as bioorthogonal chemical reporters. J Am Chem Soc 134(45):18638–18643. doi:10.1021/ja3060436
Yang J, Seckute J, Cole CM, Devaraj NK (2012) Live-cell imaging of cyclopropene tags with fluorogenic tetrazine cycloadditions. Angew Chem Int Ed 51(30):7476–7479. doi:10.1002/anie.201202122
Engelsma SB, Willems LI, van Paaschen CE, van Kasteren SI, van der Marel GA, Overkleeft HS, Filippov DV (2014) Acylazetine as a dienophile in bioorthogonal inverse electron-demand diels-alder ligation. Org Lett 16(10):2744–2747. doi:10.1021/ol501049c
Knall AC, Slugovc C (2013) Inverse electron demand Diels-Alder (iEDDA)-initiated conjugation: a (high) potential click chemistry scheme. Chem Soc Rev 42(12):5131–5142. doi:10.1039/c3cs60049a
Kämpchen T, Massa W, Overheu W, Schmidt R, Seitz G (1982) Zur Kenntnis von reaktionen des 1,2,4,5-tetrazin-3,6-dicarbonsäure-dimethylesters mit nucleophilen. Chem Ber 115(2):683–694. doi:10.1002/cber.19821150228
Kamber DN, Liang Y, Blizzard RJ, Liu F, Mehl RA, Houk KN, Prescher JA (2015) 1,2,4-triazines are versatile bioorthogonal reagents. J Am Chem Soc 137(26):8388–8391. doi:10.1021/jacs.5b05100
Horner KA, Valette NM, Webb ME (2015) Strain-promoted reaction of 1,2,4-triazines with bicyclononynes. istry. Chem A Eur J 21(41):14376–14381. doi:10.1002/chem.201502397
Patterson DM, Nazarova LA, Prescher JA (2014) Finding the right (Bioorthogonal) chemistry. ACS Chem Biol 9(3):592–605. doi:10.1021/cb400828a
Beal DM, Jones LH (2012) Molecular scaffolds using multiple orthogonal conjugations: applications in chemical biology and drug discovery. Angew Chem Int Ed 51(26):6320–6326. doi:10.1002/anie.201200002
Tunca U (2014) Orthogonal multiple click reactions in synthetic polymer chemistry. J Polym Sci Pol Chem 52(22):3147–3165. doi:10.1002/pola.27379
Greiss S, Chin JW (2011) Expanding the genetic code of an animal. J Am Chem Soc 133(36):14196–14199. doi:10.1021/ja2054034
Bianco A, Townsley FM, Greiss S, Lang K, Chin JW (2012) Expanding the genetic code of Drosophila melanogaster. Nat Chem Biol 8(9):748–750. doi:10.1038/Nchembio.1043
Chang PV, Dube DH, Sletten EM, Bertozzi CR (2010) A Strategy for the selective imaging of glycans using caged metabolic precursors. J Am Chem Soc 132(28):9516–9518. doi:10.1021/ja101080y
King M, Wagner A (2014) Developments in the field of bioorthogonal bond forming reactions-past and present trends. Bioconjugate Chem 25(5):825–839. doi:10.1021/bc500028d
McKay CS, Finn MG (2014) Click chemistry in complex mixtures: bioorthogonal bioconjugation. Chem Biol 21(9):1075–1101. doi:10.1016/j.chembiol.2014.09.002
Spicer CD, Davis BG (2014) Selective chemical protein modification. Nat Commun. doi:10.1038/ncomms5740
Boutureira O, Bernardes GJL (2015) Advances in chemical protein modification. Chem Rev 115(5):2174–2195. doi:10.1021/cr500399p
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Carell, T., Vrabel, M. Bioorthogonal Chemistry—Introduction and Overview. Top Curr Chem (Z) 374, 9 (2016). https://doi.org/10.1007/s41061-016-0010-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41061-016-0010-x