Abstract
Background
Additional antibiotic options are needed to treat bone and joint infections caused by penicillin-resistant Gram-positive pathogens.
Objective
This subanalysis of the Telavancin Observational Use Registry (TOUR™) aimed to record real-world telavancin usage patterns in patients with bone and joint infections treated with telavancin.
Methods
TOUR was a multicenter observational-use registry study conducted at 45 US sites between January 2015 and March 2017. Patient characteristics, infection type, infecting pathogen(s), previous treatment, telavancin dosing and duration, clinical response, and adverse event data were collected by retrospective medical chart reviews. As such, inclusion/exclusion criteria were limited, and any patient receiving at least one dose of telavancin at the discretion of the treating physician was eligible. Patients were assessed as either positive clinical response, failed treatment, or indeterminate outcome.
Results
Of the 1063 patients enrolled in TOUR, 27.4% (291/1063) were patients with bone and joint infections including osteomyelitis (with or without prosthetic material), acute septic arthritis, and prosthetic joint infections. Most of these patients had osteomyelitis without prosthetic material (191/291; 66.0%). Among patients assessed at the end of treatment, 211/268 (78.7%) achieved a positive clinical response, 26/268 (9.7%) failed treatment, and 31/268 (11.6%) had an indeterminate outcome. The most frequent pathogen was methicillin-resistant Staphylococcus aureus (110/291; 37.8%). The median (interquartile range [IQR as Q1, Q3]) telavancin dose was 750.0 mg (IQR, 750, 750 mg) or 8.2 mg/kg (IQR, 6.8, 9.7 mg/kg) administered for a median of 26 days (IQR, 12, 42 days). These assessments were recorded in the registry ≥ 30 days after the last dose of telavancin was administered.
Conclusions
Real-world data from the TOUR study show that clinicians are using once-daily telavancin with positive clinical outcomes for the treatment of bone and joint infections caused by Gram-positive pathogens.
Clinical Trial Registration
This trial was registered with ClinicalTrials.gov (NCT02288234) on 11 November, 2014.
Similar content being viewed by others
Clinicians are using once-daily telavancin with positive clinical outcomes for the treatment of bone and joint infections caused by Gram-positive pathogens |
Telavancin is generally well tolerated in patients with bone and joint infections |
This subanalysis suggests that telavancin is a promising and viable option for patients with bone and joint infections due to Staphylococcus aureus including methicillin-resistant S. aureus |
1 Background
Bone and joint infections caused by methicillin-resistant Staphylococcus aureus (MRSA) or penicillin-resistant Enterococcus are preferentially treated with vancomycin and require prolonged treatment [1,2,3]. However, data from the literature are unclear on the ability of vancomycin to penetrate bone efficiently. Biofilm development in these infections can also cause the clinical quiescence of osteomyelitis, complicating effective therapy [2, 4,5,6,7]. Furthermore, vancomycin dosing requires therapeutic drug concentration monitoring, and this may be highly variable. This imposes a significant burden on patients who require prolonged treatment [2, 8, 9]. There is thus an unmet need for antibiotics to treat bone and joint infections caused by penicillin-resistant Gram-positive pathogens.
Telavancin is a once-daily, parenterally administered, lipoglycopeptide antibacterial agent for the treatment of Gram-positive pathogens, including methicillin-susceptible S. aureus (MSSA) and MRSA [10]. Telavancin has demonstrated noninferiority to vancomycin in pneumonia treatment of both MSSA and MRSA [11, 12]. The Telavancin Observational Use Registry (TOUR™) was conducted to record real-world telavancin usage patterns, including population characteristics and clinical outcomes of patients treated with telavancin for infections with Gram-positive pathogens [13, 14]. Although many patients in TOUR were treated for the approved indications of complicated skin and skin structure infections and hospital-acquired and ventilator-associated bacterial pneumonia, participating physicians elected to use telavancin for other types of infection. Over a quarter (27.4%) of the telavancin use in this registry was for bone and joint infections; this registry study subanalysis evaluates the clinical characteristics and outcomes for these TOUR patients.
2 Methods
2.1 Study Design and Oversight
The design of TOUR has been described in detail [13]. TOUR was a multicenter, observational-use registry study conducted at 45 US hospitals or outpatient infusion centers between January 2015 and March 2017. All treatment decisions and clinical assessments were based on the judgment of the treating physician and not mandated by a registry study design or protocol. All data were obtained by retrospective medical chart reviews. The sponsor did not provide the study drug to patients; there were no comparator groups; and there were no restrictions on concomitant treatments.
The registry study was conducted in compliance with the International Society for Pharmacoepidemiology Guidelines for Good Pharmacoepidemiology Practice, the Declaration of Helsinki and its amendments, and the Health Insurance Portability and Accountability Act of 1996. Institutional review board waiver or approval was obtained consistent with local regulations prior to collection of patient data at each site. As informed consent was not obtained, only de-identified information was collected in accordance with Section 164.514 of the Health Insurance Portability and Accountability Act Privacy Rule.
2.2 Patient Population
Patients who received one or more doses of telavancin as part of clinical care, excluding interventional research studies or clinical trials, after 1 January, 2015 were eligible for inclusion at the treating physician’s discretion. This subgroup analysis focused on patients with bone and joint infections, including but not limited to osteomyelitis with or without prosthetic material, acute septic arthritis, and prosthetic joint infections.
2.3 Data Collection
Data of interest were obtained by retrospective medical chart reviews and entered into electronic case report forms by qualified site personnel ≥ 30 days after administration of the last dose of telavancin. Information captured included patient characteristics; infection type; infecting pathogen(s); whether infection was previously treated unsuccessfully and which antibiotics were used; health resource utilization, including surgical and medical interventions, hospitalizations, and intensive care unit stays; telavancin dosing and duration of treatment; clinical response; and adverse events (AEs) of interest, including renal AEs, any AEs leading to discontinuation of telavancin treatment, and AEs with a fatal outcome.
2.4 Data Analysis
Population size was based on patient recruitment over a planned period of approximately 3 years. All analyses except clinical response at the end of telavancin treatment (EOT) included all patients who received one or more doses of telavancin. The analysis set for clinical response at EOT included only patients with an available assessment at EOT. No formal hypothesis or statistical significance testing was performed. The qualitative and quantitative nature of the data collected and characteristics of the population were analyzed descriptively. Efficacy was assessed from clinical response at the EOT and designated as positive, failed, or indeterminate. Positive clinical response included patients who were cured or improved to step-down oral therapy. Cure was defined as the resolution of signs and symptoms of infection or no need for additional antibiotic therapy, or clearance of infection demonstrated by a negative culture. Partial resolution of clinical signs and symptoms of infection or the need for additional oral antibiotic therapy was designated as improvement to step-down therapy. Treatment failure was defined as an inadequate response to therapy, including worsening or new signs or symptoms of infections; need to change antibiotic therapy before planned completion of telavancin treatment; or positive culture at the EOT. Response was recorded as indeterminate when there was insufficient information to determine a positive clinical response or failure. Clinical response was recorded at post-treatment assessment 7–30 days after EOT.
Safety was assessed from AEs and change in renal function. Collection of AEs was limited to events of interest. A renal AE was defined as any untoward medical occurrence judged by the investigator to be related to the kidney, urinary tract, or renal function. Change in renal function was measured as change in serum creatinine and serum creatinine clearance (CrCl) estimated by the Cockcroft–Gault method. Changes in serum creatinine and CrCl were calculated from baseline to last value and worst value on treatment in all patients with available measurements; changes in CrCl were also calculated in patients with available measurements who received telavancin for > 21 days.
Results were reported as mean with standard deviation, median with interquartile range (IQR as Q1, Q3), and number with proportion, as appropriate. All AE and medical history verbatim terms were recorded and coded using the Medical Dictionary for Regulatory Activities (MedDRA®), Version 17.1. MedDRA® terminology is the international medical terminology developed under the auspices of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. The MedDRA® trademark is owned by the International Federation of Pharmaceutical Manufacturers and Associations on behalf of the International Conference on Harmonisation. Concomitant medications were coded using the World Health Organization Drug Dictionary, September 2014. Analysis was performed using SAS® Version 9.2 or higher (SAS Institute, Cary, NC, USA).
3 Results
3.1 Patient Demographics and Clinical Characteristics
From January 2015 to March 2017, TOUR enrolled 291 patients with bone and joint infections. Four of these patients died during the registry study; only one death was considered possibly related to telavancin. Clinical response assessments at EOT were available for 268/291 (92.1%) patients; disposition of patients with missing or undocumented EOT assessments is summarized in the Electronic Supplementary Material (ESM). Baseline demographics and clinical characteristics of the enrolled patients are presented in Table 1. The majority of patients were White (245/291; 84.2%), male (192/291; 66.0%), and < 65 years of age (210/291; 72.2%). In total, 81/291 (27.8%) patients were ≥ 65 years of age, of whom 26/81 were ≥ 75 years of age. Hypertension and type 2 diabetes mellitus were the most common comorbidities. Baseline CrCl was < 80 mL/min in 35/291 patients and missing for 126/291 (43.3%) patients. At baseline, 29/291 (10.0%) patients had chronic renal failure, and 6/291 (2.1%) patients were undergoing dialysis. The most frequent infection subtype was osteomyelitis without prosthetic material (191/291; 65.6%). In the majority of patients (213/291; 73.2%), the location of the primary infection site was the lower extremity; 43/291 (14.8%) infections were in the upper extremity; 26/291 (8.9%) in the back or torso; and 6/291 (2.1%) in the head and neck; one patient (0.3%) had a primary blood infection. Vertebral infections were not recorded systematically; based on verbatim infection sites reported, approximately 20/291 patients had osteomyelitis or septic arthritis affecting vertebrae or intervertebral discs. Surgical and medical procedures performed for treatment included wound irrigation, debridement, synovectomy, wound vacuum-assisted closure use, incision and drainage, joint or bursa aspiration, arthroscopic joint washout, removal of external fixators and implanted devices, spacer placement or exchange, skin grafting, bone grafting, and amputation; this information was also reviewed from verbatim reports as opposed to systematically recorded information for this study.
3.2 Isolated Pathogens
Baseline bacterial culture identified one or more pathogens in 208/291 (71.5%) patients. Pathogens included Gram-positive-only organisms in 178/291 (61.2%) patients, multiple Gram-positive-only organisms in 16/291 (5.5%) patients, and mixed Gram-positive/negative infection in 24/291 (8.2%) patients. In 83/291 (28.5%) patients, no pathogen was isolated or documented at baseline; only Gram-negative organisms or other pathogens for which telavancin is not indicated were identified in 11/291 (3.8%) patients. The most frequently isolated pathogens for which telavancin is indicated were MRSA (110/291; 37.8%), MSSA (43/291; 14.8%), and coagulase-negative Staphylococcus (29/291; 10.0%) (Table 2). Of the subjects with a minimum inhibitory concentration assessment available, the minimum inhibitory concentration of vancomycin was ≥ 1 µg/mL in 18/22 patients with MRSA and 13/14 patients with MSSA, and vancomycin-intermediate S. aureus (VISA) was isolated from one patient (0.3%) (Table 2).
3.3 Prior Antibiotic Use
Telavancin was prescribed as first-line therapy in 83/291 patients and second-line or greater therapy in 208/291 patients. The most frequently used prior antibiotics in the latter population were vancomycin (95/208; 45.7%), daptomycin (40/208; 19.2%), and trimethoprim/sulfamethoxazole (23/208; 11.1%) (Table 3). Additional antibiotics with activity against Gram-positive pathogens were administered during telavancin treatment in 8/291 patients; this included four patients receiving ceftriaxone and one patient each receiving piperacillin/tazobactam, levofloxacin, gentamicin, and daptomycin. No patients received vancomycin during telavancin treatment.
3.4 Care Settings
Nearly 70% of patients started telavancin treatment in outpatient settings (203/291, 69.8%), including outpatient infusion centers and outpatient infusion clinics. Hospital settings where telavancin was initiated included the emergency department (1/291 patients); intensive care unit (1/291 patients); and non-emergency room, non-intensive care unit hospital units (70/291 [24.1%] patients). Patients who started treatment as non-emergency room, non-intensive care unit inpatients spent a median of 8 days (range 2–88 days) in the hospital, including time before telavancin initiation.
3.5 Prescribing Patterns
Telavancin was administered at a median dose of 750.0 mg (IQR, 750, 750 mg) or 8.2 mg/kg (IQR, 6.8, 9.7 mg/kg) of actual body weight for a median duration of 26 days (IQR, 12, 42 days). The average daily dose of telavancin was < 750 mg in 58/291 (19.9%) patients, 750 mg in 180/291 (61.9%), > 750 to < 1000 mg in 21/291 (7.2%), 1000 mg in 18 (6.2%), and > 1000 mg in 14 (4.8%) patients. Telavancin dose was generally lower in patients with decreased CrCl relative to patients with normal renal function, consistent with dose adjustment per telavancin labeling (Table 4). These dosing decisions were made by the prescribing clinician. A total of 25/291 patients (8.6%) received telavancin dose adjustment during treatment.
3.6 Clinical Response
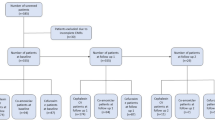
Among patients with available EOT assessment, 211/268 (78.7%) achieved a positive clinical response, 26/268 (9.7%) did not respond to treatment, and 31/268 (11.6%) had an indeterminate outcome (Fig. 1a); available outcomes in patients without EOT assessments are described in the ESM. The positive clinical response rate ranged from 75.0% (21/28) in patients with osteomyelitis with prosthetic material to 89.5% (34/38) in patients with acute septic arthritis (Fig. 1b). More than 75% of patients with either MSSA (35/41; 85.4%) or MRSA (77/99; 77.8%) had a positive clinical response to telavancin treatment (Fig. 1c); the single patient with known vancomycin-intermediate S. aureus at baseline had a clinical cure at EOT. Positive clinical responses were recorded in 95/119 (79.8%) patients with baseline CrCl ≥ 80 mL/min, 17/24 (70.8%) patients with baseline CrCl 50 to < 80 mL/min, and 7/9 (77.8%) patients with baseline CrCl 30 to < 50 mL/min (Fig. 1d). Of 211 patients with positive clinical response at EOT, 148 were cured and 63 were improved to step-down oral therapy. At the post-treatment assessment, 175/240 (72.9%) patients with available assessment were cured, 30/240 (12.5%) failed treatment, and 35/240 (14.6%) had an indeterminate response.
Clinical outcomes after telavancin treatment of bone and joint infections a overall, b by infection subtype, c by infecting pathogen, and d by baseline creatinine clearance. Positive clinical response includes patients deemed cured or improved to oral step-down therapy. aVancomycin-sensitive Enterococcus faecium (vancomycin minimum inhibitory concentration = 2 µg/mL) with methicillin-resistant Staphylococcus aureus (MRSA) [vancomycin minimum inhibitory concentration = 2 µg/mL] coinfection; Enterobacter cloacae and Citrobacter freundii also detected at baseline. bOne patient assessed at the end of treatment had creatinine clearance (CrCl) < 30 mL/min and had a positive clinical outcome; four patients assessed at the end of treatment were receiving dialysis at baseline, of whom one had a positive clinical response, two experienced treatment failure, and one had an indeterminate outcome. MSSA methicillin-susceptible Staphylococcus aureus
3.7 Safety
Treatment-emergent AEs (TEAEs) of interest occurred in 58/291 (19.9%) patients, comprising renal TEAEs in 27/291 (9.3%) patients, TEAEs leading to discontinuation of telavancin in 46/58 patients, and TEAEs leading to a fatal outcome in 4/291 (1.4%) patients (Table 5). Two of the deaths were considered unrelated to telavancin, one in a patient with lumbar discitis and concomitant bacteremia as a result of MRSA who died because of worsening lung cancer and one in a patient with bilateral prosthetic knee infection as a result of MRSA who died from respiratory arrest following a pain medication overdose. One patient with osteomyelitis of the ankle and bacteremia as a result of MRSA died because of a cardiac arrest after worsening renal failure; this death was considered possibly related to telavancin. The fourth patient with a fatal outcome had lumbar discitis with no specific pathogen recorded; neither cause of death nor its relationship to telavancin was recorded. All four patients with fatal outcomes received telavancin as second-line or greater therapy, and three patients received two or more prior antibiotics. Serious TEAEs were recorded in 6/291 (2.1%) patients (Table 5), comprising two patients with renal failure, one patient with renal failure and cardiac arrest, and one patient each with worsening lung cancer, respiratory arrest, and death from reason unrecorded. Study discontinuation because of TEAEs occurred in 16/58 patients. Only 17.5% of TEAEs (51/291) were considered possibly related to telavancin. The most common individual TEAE was renal failure (8.9%), followed by nausea (3.1%) and vomiting (2.1%); increased serum creatinine based on laboratory data was reported in three (1.0%) patients, of whom one discontinued treatment. Most TEAEs (51/58) resolved, including 22/26 cases of renal failure (Table 5).
The mean (standard deviation) change in serum creatinine from baseline was 0.13 (0.32) mg/dL for the last value on treatment and 0.25 (0.42) mg/dL for the worst value on treatment (both n = 139). Among all 137 patients with available data, mean (standard deviation) change in CrCl from baseline was − 15.0 (39.0) mL/min for the last value on treatment and − 23.7 (39.4) mL/min for the worst value on treatment; median (IQR) change in CrCl from baseline was − 7.9 (− 28.4, 0.0) mL/min for the last value on treatment and − 16.6 (− 35.1, 0.0) mL/min for the worst value on treatment. Changes in CrCl were generally similar between all patients and patients treated with telavancin for > 21 days (Table 6).
4 Discussion
Results from TOUR™ demonstrate the use of telavancin for real-world treatment of bone and joint infections caused by Gram-positive pathogens. The majority of patients had osteomyelitis without prosthetic material (191/291, 65.6%) located in the lower extremity (213/291, 73.2%) and initiated telavancin treatment in outpatient settings (203/291, 69.8%). Telavancin was administered intravenously at a median dose of 750.0 mg or 8.2 mg/kg for a median duration of 26 days, and the majority (208/291, 71.4%) received telavancin as a second-line therapy or greater after failed antibiotic treatments. More than 75% of patients assessed at EOT had positive clinical responses to telavancin treatment (211/268, 78.7%). Telavancin was generally well tolerated in patients with bone and joint infections. Renal failure occurred in 26/291 (8.9%) patients and resolved in nearly 85% of affected patients (22/26, 84.6%). Renal function measured by a change in CrCl declined slightly from baseline during telavancin treatment; however, a decline in renal function did not appear related to duration of telavancin treatment. Although vertebral osteomyelitis is of sufficient concern to warrant a specific Infectious Diseases Society of America clinical practice guideline [1], outcomes in at least 20 patients with vertebral infections in TOUR, as discovered in verbatim descriptions, could not be analyzed because infection sites were not systematically reported.
Telavancin is approved for the treatment of complicated skin and skin structure infections and hospital-acquired and ventilator-associated bacterial pneumonia but not for the treatment of bone and joint infections [10]. However, of the antibiotics recommended by the Infectious Diseases Society of America for the treatment of bone and joint infections caused by Gram-positive pathogens such as S. aureus, nafcillin, cefazolin, ceftriaxone, clindamycin, vancomycin, daptomycin, and linezolid [1,2,3], few are specifically indicated for bone and joint infections. Nafcillin and vancomycin are approved for the treatment of susceptible penicillinase-producing Staphylococci without restriction by infection type; for bone infections, the label for injectable vancomycin states only “effectiveness has been documented” [6, 15]. Cefazolin, ceftriaxone, and clindamycin are indicated for bone and joint infections caused by susceptible Gram-positive pathogens in general or S. aureus in particular [16,17,18]. The newest Infectious Diseases Society of America-recommended agents, linezolid (approved in 2000) and daptomycin (2003), are not US Food and Drug Administration approved for the treatment of bone and joint infections [19, 20]; furthermore, dalbavancin and oritavancin are also not approved for bone and joint infections [21, 22]. Ciprofloxacin, approved in the USA in 1987, may be the last antibiotic to date to receive a specific indication for bone and joint infections, and is only approved for those caused by certain Gram-negative pathogens [23]. Moreover, the resistance and susceptibility patterns over time and geography must be accounted for in effective prescribing decisions. Given the treatment options available, clinicians often elect to use antibiotics that are not specifically approved for bone and joint infections in patients for whom other antibiotics are ineffective or unsuitable.
Concomitant conditions also impact antibiotic diffusion into bone tissue. For instance, vancomycin diffuses into bone more poorly when ischemia is present. In contrast, linezolid and clindamycin achieve adequate treatment concentrations in ischemic lower limbs [6]. Compelling in vitro data indicating bone penetration also suggest telavancin is a viable option, especially for hospital-acquired MRSA [6, 24, 25]. The extended stability of telavancin renders it appropriate and convenient for use in elastomeric devices for self-administration at home [26]. In addition, more than half of the subjects in this subanalysis initiated telavancin in an outpatient setting. Moreover, standard dosing for telavancin is every 24 h with no monitoring of serum concentrations required, only 48- to 72-h interval monitoring or more frequently, if clinically indicated, of serum creatinine for safety [10].
Recent data on the treatment of bone and joint infections come largely from retrospective and/or observational studies such as TOUR. The retrospective, observational Cubicin® Outcomes Registry and Experience (CORE®) study in the USA identified 82 patients with device-associated osteomyelitis treated with daptomycin, of whom 48 had available follow-up information; 22/27 (82%) of patients with prosthetic joint infection and 18/21 (86%) patients with other hardware-associated osteomyelitis had a cure or improvement with daptomycin treatment [27]. Patients with osteomyelitis and orthopedic device infections in a retrospective European registry (EU-CORESM) were cured or improved after daptomycin treatment in 522/638 (81.8%) cases [28]. As in this TOUR subanalysis, Schroeder et al. reported that telavancin was most often used as a second-line option, for example, after daptomycin or vancomycin therapy failures [29]. Vancomycin remains the recommended first-line treatment and standard of care for oxacillin-resistant Staphylococci and penicillin-resistant Enterococcus species [3]. Registry studies such as TOUR can thus provide real-world data on the use of antibiotics to treat bone and joint infections when clinical trials are not feasible.
The present registry study has several limitations. Enrollment decisions were made by individual site clinicians, introducing potential patient selection bias. Only data available from retrospective chart review were captured, and not all data of interest were recorded. The purpose of this observational retrospective study was to learn and provide insight on how telavancin is being used in the real world and the efficacy of these different indications. Because of the retrospective nature of this study, the registry includes limited details and laboratory values necessary for the interpretation and assessment specific to bone and joint infections. The reasons for dosage decisions, treatment changes from prior antibiotic therapy to telavancin or from telavancin to another antibiotic were not recorded, and it is unclear whether prior antibiotics were discontinued because of a lack of efficacy, toxicity, or simplification of the antibiotic regimen. Similarly, other antibiotics used within 2 days prior to 2 days after the start of telavancin treatment were recorded as concurrent use, but administration dates were not consistently recorded. There were eight subjects for whom concurrent antibiotics with Gram-positive coverage were documented but whether these were truly “concurrent” or being tapered prior to telavancin remains unknown. Clinical response data at EOT were unavailable for many patients, which could bias the interpretation of clinical outcomes. Only AEs of interest were captured, and AE reporting was also inconsistent; for example, it was unclear whether increased blood creatinine reported in three patients was related to renal failure or a separate event. There were two death cases that were assessed by the investigators as possibly related to the study drug, a case of cardiac arrest and a case of worsening renal failure. No other detail was provided regarding the relatedness. It is recommended that renal function be monitored during telavancin therapy [10]. Such limitations are intrinsic to retrospective observational studies, yet the data from TOUR still provide valuable information on the clinical characteristics and treatment outcomes of patients with these challenging infections.
5 Conclusions
Real-world data from TOUR offer insights into clinicians’ use of telavancin to treat bone and joint infections caused by Gram-positive pathogens. The positive clinical response rates and safety results reported suggest that telavancin may be a useful treatment option for patients with bone and joint infections when other antibiotics are unsuitable.
References
Berbari EF, Kanj SS, Kowalski TJ, et al. 2015 Infectious Diseases Society of America (IDSA) clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adults. Clin Infect Dis. 2015;61(6):e26-46.
Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18-55.
Osmon DR, Berbari EF, Berendt AR, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):e1-25.
Calhoun JH, Manring MM, Shirtliff M. Osteomyelitis of the long bones. Semin Plast Surg. 2015;23:59–72.
Zimmerli W, Sendi P. Orthopedic biofilm infections. APMIS. 2017;125(4):353–64.
Thabit AK, Fatani DF, Bamakhrama MS, et al. Antibiotic penetration into bone and joints: an updated review. Int J Infect Dis. 2019;81:128–36.
Tice AD, Hoaglund PA, Shoultz DA. Risk factors and treatment outcomes in osteomyelitis. J Antimicrob Chemother. 2003;51(5):1261–8.
Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-system Pharmacists, the Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis. 2020;71(6):1361–4.
Norris AH, Shrestha NK, Allison GM, et al. 2018 Infectious Diseases Society of America clinical practice guideline for the management of outpatient parenteral antimicrobial therapy. Clin Infect Dis. 2019;68(1):e1-35.
Vibativ® (telavancin) [package insert]. Nashville, TN: Cumberland Pharmaceuticals, Inc.; 2020.
Rubinstein E, Lalani T, Corey GR, et al. Telavancin versus vancomycin for hospital-acquired pneumonia due to gram-positive pathogens. Clin Infect Dis. 2011;52(1):31–40.
Thabit AK, Nicolau DP, Kuti JL. In vitro pharmacodynamics of human simulated exposures of telavancin against methicillin-susceptible and -resistant Staphylococcus aureus with and without prior vancomycin exposure. Antimicrob Agents Chemother. 2015;60(1):222–8.
Bressler AM, Hassoun AA, Saravolatz LD, et al. Clinical experience with telavancin: real-world results from the Telavancin Observational Use Registry (TOUR). Drugs Real World Outcomes. 2019;6(4):183–91.
Reilly J, Jacobs MA, Friedman B, et al. Clinical experience with telavancin for the treatment of patients with bacteremia and endocarditis: real-world results from the Telavancin Observational Use Registry (TOURTM). Drugs Real World Outcomes. 2020;7(3):179–89.
Nafcillin for injection [package insert]. Schaumburg, IL: SAGENT Pharmaceuticals; 2020.
Cleocin phosphate® (clindamycin injection) and (clindamycin injection in 5% dextrose) [package insert]. New York, NY: Pharmacia and Upjohn Co.; 2020.
Ceftriaxone for injection [package insert]. Schaumberg, IL: SAGENT Pharmaceuticals; 2020.
Cefazolin for injection [package insert]. Lake Forest, IL: Hospira, Inc.; 2020.
Zyvox® (linezolid) injection, for intravenous use; tablets, for oral use; for oral suspension [package insert]. New York, NY: Pharmacia & Upjohn Co.; 2020.
Cubicin® (daptomycin for injection) [package insert]. Whitehouse Station, NJ: Merck & Co., Inc.; 2020.
Dalvance® (dalbavancin for injection) [package insert]. Madison, NJ: Durata Therapeutics Inc., an Allergan affiliate; 2018.
Orbactiv® (oritavancin) for injection [package insert],. Lincolnshire, IL: Melinta Therapeutics, Inc.; 2019.
Cipro® (ciprofloxacin hydrochloride) tablet, for oral use; for oral suspension [package insert]. Wayne, NJ: Bayer Healthcare Pharmaceuticals Inc.; 2020.
Landersdorfer CB, Bulitta JB, Kinzig M, et al. Penetration of antibacterials into bone. Clin Pharmacokinet. 2009;48(2):89–124.
Yin L-Y, Calhoun JH, Thomas TS, et al. Efficacy of telavancin in the treatment of methicillin-resistant Staphylococcus aureus osteomyelitis: studies with a rabbit model. J Antimicrob Chemother. 2008;63(2):357–60.
Sand P, Aladeen T, Kirkegaard P, et al. Chemical stability of telavancin in elastomeric pumps. Curr Ther Res Clin Exp. 2015;77:99–104.
Hermsen ED, Mendez-Vigo L, Berbari EF, et al. A retrospective study of outcomes of device-associated osteomyelitis treated with daptomycin. BMC Infect Dis. 2016;16:310.
Malizos K, Sarma J, Seaton RA, et al. Daptomycin for the treatment of osteomyelitis and orthopaedic device infections: real-world clinical experience from a European registry. Eur J Clin Microbiol Infect Dis. 2016;35(1):111–8.
Schroeder CP, Van Anglen LJ, Dretler RH, et al. Outpatient treatment of osteomyelitis with telavancin. Int J Antimicrob Agents. 2017;50(1):93–6.
Acknowledgements
The authors thank all of the patients and investigators who were involved with TOUR, without whom this study would not have been possible; Nancy Havrilla, MS, RN, for her efforts in coordinating TOUR; and Heidi Goldstein, a consultant for Theravance Biopharma US, Inc., for data validation and quality control. Editorial and medical writing support was provided by Judy Phillips, DVM, PhD, of AlphaBioCom, LLC, and Elizabeth Conrad, PhD, of Cumberland Pharmaceuticals, Inc. and funded by Theravance Biopharma R&D, Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Theravance Biopharma R&D, Inc.
Conflict of interest
Charles R. Sims has received research support and speaker honoraria from Theravance Biopharma R&D, Inc. Adam M. Bressler has received fees for participating in advisory boards and speaker bureaus for Theravance Biopharma R&D, Inc. Donald R. Graham has received grants or research support from Apex Bioscience, Aronex, Inc., Arpida, Artisan Pharmaceutical, Astra Zeneca, Aventis, Basilea, Inc., Bayer Corp., Biocryst, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, CEM-102, Centocor, Cerexa, Chiron, Inc., Cubist, Inc., Forest Pharmaceuticals, Genomics, Gilead, GlaxoSmithKline, Incyte Corp., InsMed, Johnson & Johnson, Liposomal Company, Inc., The Medicines Company, Merck & Co., Miles Pharmaceuticals, Novartis Pharmaceuticals, Optimer Pharmaceuticals, Ortho-McNeil, Parke-Davis, Peninsula Pharmaceuticals, Pfizer, Rib-X, Roche Pharmaceuticals, R. W. Johnson, Inc., Salix Pharmaceuticals, Sanofi Pasteur, Schering-Plough, Sequus, Inc., Serono, Targanta, Theravance Biopharma R&D, Inc., Tibotec, Trius Therapeutics, Vertex, and Xoma Corp., and consulting fees from Bristol-Myers Squibb, City of Springfield, Oscient Pharmaceuticals, Memorial Medical Center, Illinois Department of Human Services, Division of Mental Health, Rhone-Poulenc Rorer, Saint John’s Hospital, Sangamon County Department of Public Health, Illinois, Vibra Hospital, Springfield, and various home care programs, nursing homes, and tuberculosis programs. Melinda K. Lacy and David A. Lombardi DL and ML are employees of Theravance Biopharma US, Inc., and may hold stock. Bibiana Castaneda-Ruiz is a former employee of Theravance Biopharma US, Inc., and may hold stock.
Ethics approval
The Copernicus Group Independent Review Board approved the TOUR observational study protocol on 29 December, 2015.
Consent to participate
This was an observational use study with data collected via retrospective medical chart reviews, therefore signed informed consent from the patient was not required. Only de-identified information was collected in accordance with Section 164.514 of the Health Insurance Portability and Accountability Act Privacy Rule.
Consent for publication
Not applicable.
Availability of data and material
Cumberland Pharmaceuticals Inc. (and its affiliates) will not be sharing individual de-identified patient data or other relevant study documents.
Code availability
Not applicable.
Author contributions
All authors reviewed, read, and approved the final manuscript. BC and ML provided critical analysis of the data and were major contributors. CS, AB, and DG were investigators who contributed patient data to TOUR and provided a critical analysis of the data. DL performed the statistical analysis.
Additional information
Bibiana Castaneda-Ruiz: Former employee of Theravance Biopharma US, Inc.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Sims, C.R., Bressler, A.M., Graham, D.R. et al. Real-World Clinical Use and Outcomes of Telavancin for the Treatment of Bone and Joint Infections: Results from the Telavancin Observational Use Registry (TOUR™). Drugs - Real World Outcomes 8, 509–518 (2021). https://doi.org/10.1007/s40801-021-00255-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40801-021-00255-6