Abstract
Stem cells reside in a complex milieu during development, or in adult tissues, as well as in culture conditions. Their decision to differentiate, self-renew, or migrate is a result of an integrated response to extracellular stimuli, which are chemical, physical, and mechanical in nature. In recent years, research has shown that the mechanical properties of the microenvironment can regulate a variety of stem cell phenotypes by activating intracellular signal transduction leading to transcription. Many of these signaling pathways are primarily involved in mechanotransduction, suggesting that mechanical cues, particularly the rigidity and topographical architecture of the extracellular matrix directly regulate stem cell behavior. Novel bioengineering tools have made it possible for the first time to systematically and quantifiably understand the role of mechanical cues in stem cell biology. However, it is necessary to investigate activation of mechanotransduction in the context of other signals to which cells respond. How cells integrate complex presentation of signals, including mechanical cues, to formulate a decision will increase our understanding of fundamental stem cell biology, as well as inform future therapeutic applications in regenerative medicine.
Similar content being viewed by others
Introduction
Recent years have brought an increased focus on the mechanical properties of cellular microenvironment as an important determinant in deciding their phenotypes, including the fate of stem cells [1]. Mechanical properties of the microenvironment, unlike other chemical signals, act as cues that are long ranging both in time and space. Mechanical cues from the extracellular matrix (ECM), in absence of injury, only change their properties gradually over time, as well as over long physical ranges comprising of many cell lengths. Therefore, mechanical cues by their very nature are persistent over long periods of time and continue to provide similar stimulation to cells when they migrate over sufficiently long distances. These cues comprise of the rigidity, as well as the structural topography of the ECM, and a large body of recent literature implicates their role in regulating various cellular processes in stem cells, including maintenance of their potency, division, differentiation, metabolism, migration, and their interaction with other cells [2]. Mechanical cues, owing to their persistence and continuity, constitute an essential component of any signaling event and provide a constant context to all extracellular signaling. However, it is only in recent years that their role in regulating stem cell phenotype has received the attention it deserved. The increased focus has been revealing: we not only know that stem cells depend crucially on the mechanical inputs from their microenvironment to regulate nearly all their phenotypes but also many mechanosensors, as well as mechanotransducers have been discovered that mediate the extracellular mechanical cues to intracellular transcription and thereby regulate cell behavior. The accumulated work has given birth to a new field of mechanobiology, which has generated an active interest in deeper understanding of the role of mechanics in regulating stem cell phenotypes, as well as in developing therapeutic and translational tools in tissue engineering that harness the gained knowledge from mechanoregulation of stem cell behavior [3–6].
In this brief review, we will survey the recent advances in our understanding of mechanobiology of stem cells, as well as overview our current understanding of the signaling pathways involved in mechanical regulation of stem cell behavior. We will then place mechanical cues in the context of other cues sensed by stem cells and understand how cellular decision is based on a combinatorial inputs of signals, that nearly always include mechanical cues.
Mechanical Instructions from the Microenvironment
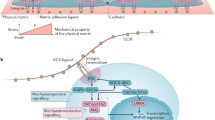
Adult stem cells reside in a complex microenvironment, interacting with the ECM to which they attach in a neighborhood comprising other cell types exchanging soluble and insoluble biochemical signals and responding to them [7, 8]. Extensive research had revealed the complexity of cell-ECM interactions, the diversity of ECM-induced signaling via integrins, and the intracellular signaling pathways they activate. ECM constitution varies significantly between tissues and consists of a variety of proteins, proteoglycans, carbohydrates, and their derivatives, creating diverse chemical and mechanical properties [9, 10]. ECM can therefore signal to cells at different levels, though never exclusively: as chemical cues via the specific motifs present in its molecules that are recognized by the cognate receptors expressed on stem cell membrane, as scaffolds to which soluble paracrine signals secreted by other cells attach to, and as mechanical cues owing to their physical and ultrastructural architecture (Fig. 1). ECM signaling is therefore both chemical and mechanical in nature, and it is only in recent years that the specific role of the mechanical inputs from ECM have been explored in detail. Mechanical rigidity of the substratum itself can regulate stem cell fate, allowing mesenchymal stem cells which are of mesodermal lineage to assume fate as diverse as neuronal, adipogenic, osteogenic, and skeletal muscle by altering the rigidity of the substratum to match the measured rigidity of the respective tissue types [11]. In our own work, we found that cardiosphere-derived cells (CDCs), a heterogeneous collection of cells isolated from myocardial explants or biopsies with known cardiovascular potential, were exquisitely sensitive to the rigidity as well as topography of the underlying matrix [12•]. CDCs assumed endothelial fate in matrix with rigidity matching that of myocardium while showed cardiomyogenic differentiation when cultured on substrates mimicking the ultrastructure of the heart collagen matrix at nanoscale. Various reports now indicate that mechanical cues presented by the substrata could directly regulate stem cell fate, as well as mediate other stem cell phenotypes (Table 1).
Cues from the microenvironment are complex and combinatorically processed by stem cells. Schematic showing the integration of chemical and mechanical signals from the ECM via a range of membrane bound mechanosensor, which activate intracellular signal transduction resulting in transcriptional control of various stem cell phenotypes
However, it is important to note that mechanical cues do not act in exclusion to the chemical signals from ECM which signal stem cells via integrin-mediated signaling. When cells sense the ECM as a cue via integrins, the information conveyed intracellularly is both chemical and mechanical in nature. Mechanosensing can happen exclusively via other receptors, feeding into alternative signaling modules, but stem cell decision is dependent on integration of a variety of extracellular signals, which include both the chemical and mechanical signals from ECM, as well as other signals from the microenvironment. Therefore, while research efforts are necessary to decouple the various aspects of ECM-mediated signaling to stem cells in regulating lineage-specific phenotypic response, it would also be beneficial to understand the role of mechanical signaling in the context of other types of signals, e.g., growth factor signals from neighboring cells, oxygen and nutrient availability from blood vessels, and stress cytokines from injury or death in the immediate neighborhood. Whether the persistently present mechanical signals regulate the permissiveness of stem cells to adequately respond to these non-mechanical signals and could alteration in mechanical properties of the ECM limit the potential of stem cells to respond have been not understood in detail.
Elasticity of Matrix
In recent years, a large body of reports demonstrates that stem cell fate, as well as other phenotypes could be directly controlled by the elasticity (or rigidity) of the matrix that they are cultured on. Most untransformed cell types need to be attached to the matrix for continuous survival. The adhesion is mediated by integrin molecules that recognize specific motifs in cells that match the rigidity of the substrates they adhere to and by generating traction force intracellularly by their actomyosin cytoskeletal assembly, which also acts as mediator of intracellular mechanotransduction. The substrate proportionately deforms and thereby exerts force on the cells, and this force is sensed to initiate mechanotransduction via a variety of transmembrane mechanosensors, more and more of which are now being identified. Mesenchymal stem cells are known to differentiate between cell types of the mesodermal lineages by soluble factors provided within the medium. However, when mesenchymal cells were cultured on substrata matching the elasticity of different tissue types, they remarkably differentiated into resident cell types within those tissues [55]. Fate of other stem cell types have been shown to be dependent on matrix elasticity. It was reported that reduction of matrix rigidity could promote cardiomyocytes dedifferentiation (Fig. 2a), as well as proliferation and clonal expansion [56]. We also found that c-kit+ cells within a population of cardiosphere-derived cells rapidly expanded and differentiated into endothelial cells when cultured on a matrix matching the rigidity of heart tissue (Fig. 2b) [12•]. Cardiovascular organoid formation was found to be modulated by matrix rigidity by embryoid bodies [58]. Angiogenic signaling of mesenchymal stem cells was also found to be directed by matrix composition and mechanics [31]. Cardiac differentiation of mouse and human embryonic stem cells was found to be crucially dependent on an appropriate rigidity in the microenvironment [59]. Fate decisions of embryonic stem cells are found to be regulated by mechanics [60]. Similarly, muscle stem cell fate in vivo was dependent on their culture on compliant matrix in vitro [61].
Stem cells fate is dependent on rigidity of their dynamic microenvironment. a Compliant matrices induce cardiomyocytes to dedifferentiate; shown are cardiomyocytes expressing Nkx2.5, cTnT, and [56] dTomato marking cells that were Myh6+; cells that are dTomato+/cTnT− exhibit dedifferentiation (permission to reprint obtained from Elife) [56]. b Cardiosphere-derived cells cultured on a compliant substratum (MRS) compared to glass integrate into native blood vessels and assume CD31+ endothelial phenotype after transplantation into rat heart (permission to reprint obtained from the American Association for the Advancement of Science) [12•]. c Graph showing the dynamics of change in mechanical stiffness of different tissues during development in a chick embryo (permission to reprint obtained from Cell Press) [57]
Mechanical rigidity is mostly persistent but can change during development, aging, or in response to remodeling (Fig. 2c) [62]. Within an adult tissue after injury, resident stem cells divide and may differentiate into the cells belonging to the tissue type partly in response to the specific range of rigidity of the tissue. However, most tissue types contain a variety of somatic cell types to which the adult stem cell can terminally differentiate into. Heart, for example, consists of cardiac fibroblasts, cardiomyocytes of different nature, smooth muscle cells, and endothelial cells. Therefore, while rigidity itself could be sufficient to direct differentiation of stem cells, therefore, other chemical cues emanating from the site of injury or paracrine signaling from neighboring cells create an important physiological context within which the role of mechanical signal should be interpreted. Having solved a biological problem reductively with sufficient detail, it is imperative to consider if individual signals could combinatorially elicit response different from their additive application.
Topography of Matrix
ECM is composed of protein macromolecules, proteoglycans, and carbohydrates creating a highly diverse range of shapes and architecture. These architecture constitute sheets in basal lamina rich in laminin, fibronectin, highly arranged fiber bundles composed of collagen type I fibers, fibrillar matrix deposited by fibroblasts in a scar, or amorphous calcified formations with pits and holes in bones [2, 5]. We and others have shown that these topographical features of the ECM could regulate cell shape, proliferation, differentiation, as well as collective cell morphogenesis and tissue level functional phenotypes, e.g., directional action potential propagation across distances spanning large number of cells (Fig. 3a) [63, 65, 66••]. However, our understanding of how stem cells respond to nanotopography is limited, largely due the lack of platforms to mimic the physiological features of ECM at nanoscale in a cost-effective, long lasting in liquid cultures, and reproducible manner, as well as over large surface area necessary to perform biochemical analyses. Common technologies to create reliable nanofeatures include E-beam lithography, self-assembly of monolayers of colloids, and capillary action lithography [67]. Recent evidence has highlighted how topographical features of ECM at nanoscale could influence stem cell behavior in an active fashion. Angiogenesis and osteogenic differentiation of Mesenchymal stem cells is promoted by synergistic effect of microtopography and biochemical culture environment [68]. RhoA-mediated functions in osteoprogenitors are dependent on substrate topography [69]. Contact guidance and neuronal differentiation of human neural stem cells are enhanced by biodegradable nanotopography combined with neurotrophic signals [70]. Differentiation of mesenchymal stem cells towards osteogenic and adipogenic lineages is found to be increased by surface topography [71]. Taking advantage of the nanotopographical cues as instructive signals directing stem cell differentiation, we created large surface area substrata with spatial control of mesenchymal stem cell differentiation (Fig. 3b) [24]. The platform can therefore be used to pattern cells by directing their fate spatially to create mosaic tissues. Nanotopography influences stem cell behavior actively, and stem cell fate is dependent not only on the features but also on macroscopic arrangement of those features. Neural stem cells differentiated into neurons in aligned nanofibers, but not on randomized nanofibers (Fig. 3c) [64]. Similarly, we found that cardiosphere-derived cells increased cardiac differentiation only on aligned nanogrooves mimicking collagen fibers in heart, but not when those fibers were randomly arranged [65, 66••].
Nanotopographical architecture of ECM actively regulate stem cell phenotype. a hESC colonies adhere in markedly different fashion on nanopillared substrates with different densities, with high density ones showing rounded hESC colonies but extensive lamellipodia and fine filopodia formation compared to low and medium density of nanopillars (permission to reprint obtained from Elsevier) [63]. b hMSC sensitivity to density of nanopillars to differentiate into adipocytes (oil-red-O, red labeled) or osteocytes (alkaline phosphatase, blue labeled) could be harnessed to create large tissue constructs with spatial control over their fate (permission to reprint obtained from Elsevier) [24]. c Neural stem cells differentiated into Tuj1+ neurons when cultured on PCL nanofibers that were aligned, but not in random fibers of similar dimensions (permission to reprint obtained from Elsevier) [64]
It still is not known whether stem cells sense nanotopographical features of ECM in an exclusive way, or the shape is sensed by similar mechanisms as rigidity and integrin-mediated chemical cues, with the shape and architecture of cue presentation creating a specialized outcome. For example, stem cells adhered to a substratum mimicking collagen fibers that run parallel to each other would still sense collagen chemically and mechanically, but the specific anisotropic shape of cue presentation recruits the integrin receptors and focal adhesion formation in a highly directional fashion. Spatial heterogeneity of activation of mechanosensors would result in a non-homogeneous mechanotransduction within the cell, eliciting the response of an anisotropic cell shape, directional migration, and possibly cell division. How stem cells sense nanotopographical cues, process them intracellularly, and elicit specific responses is a research question with significant potential to excite and reveal new insights.
Mechanotransduction in a Changing Microenvironment
An often overlooked aspect in stem cell mechanobiology is the cellular processing of mechanical cues that change over time. Mechanical cues are persistent and long ranging in space, but certain physiological changes or pathologies could result in slow to rapid changes in mechanical signals. These include the effect of aging which results in gradual alterations in the chemical as well as mechanical nature of ECM [72]. Aging results in gradual, but largely irreversible changes in the extracellular matrix to which stem cells need to adapt. In the heart, for example, aging is characterized by fibrotic remodeling, increase in stiffness as well as disrepair in the highly anisotropic collagen fiber bundles at nanoscale to macroscale levels [73]. Since stem cell fate, proliferative potential, as well as other behavior is so fundamentally dependent on the mechanical cues presented by the ECM, aging could have a profound effect on the physiological potential of stem cells, limiting their regenerative capacity and possibly inducing senescence. Aging occurs in nearly all tissues of the body simultaneously, and it is still an open debate whether the limited regenerative capacity of tissues is a causative factor in aging, or the fibrotic change in microenvironment results in altered mechanical signaling, thereby reducing the regenerative potential of resident stem cells [74].
Another neglected area of our understanding is the effect wound or injury brings to intracellular mechanical signaling. Adult stem cells are typically maintained in specialized niches to respond to injury, when terminally differentiated somatic cells need to be replaced in larger numbers. Stem cell response to injury has been largely studied in the context of immune signals from the wound, or hypoxia signaling resulting from disruption of blood vessels. But most injury also results in large damage to the extracellular matrix architecture, disrupting the topography of matrix, as well as frequently changing the elasticity of the matrix [75]. Indeed, the ECM ligands may still be available at similar dosage to be sensed by integrin receptors on the cells, when the structure of ECM is in disrepair, while the mechanics is severely altered. Heart attack results in significant damage at the site of infarct, not only resulting in loss of cardiomyocytes but also significant damage to the nanotopographical architecture of the underlying collagen fibers [76]. Subsequent to the acute damage of the matrix, the site of infarct gets rapidly remodeled by fibroblasts depositing collagen which decreases the elasticity of the matrix, as well as results in permanent change to the nanotopography. How stem cells respond to acutely changing mechanical microenvironment has not been well studied, largely due to the lack of tools allowing acute changes in the presentation of mechanical cues to cells. New tools related to shape memory polymerization are now making it possible to experiment with changes in stem cells behavior in respond to rapidly changing rigidity or the structural properties of ECM [77]. A deeper understanding of mechanotransduction in rapid time scale could potentially provide avenues to treat tissue injuries that today result in permanent and irreparable damage, e.g., heart or kidney infarct.
Mechanical Cues Directly Activate Signal Transduction
Mechanical cues result in disparate outcome in cells leading to cell spreading/migration, cellular redox changes, cell differentiation, and/or apoptosis. Recent studies have shown that mechanosensing pathways not only regulate the morphology of stem cells but also regulate fate of these cells with respect to potency or differentiation into specific lineage [11, 55, 78]. These studies highlight the importance of mechanical cues in determining the stem cell biology as soluble factors had minimal effect in contradicting the mechanical signals from extra cellular environment suggesting the primacy of “force sensing” in regulating stem cells biology. Mechanical forces are also important in maintaining the pluripotency/differentiation potential of embryonic stem cells as softer microenvironment tend to favor the proliferation and maintenance of pluripotency by generating less traction [79], while stiffness and increased traction causes cell spreading and spontaneous cell differentiation [80]. These studies suggest the use of mechanical cues in design of culture platform that mimics physiological system more in tune with native stem cell environment and requires less soluble factors/cytokines to regulate stem cells biology.
Cells sense extracellular mechanical inputs though adhesion proteins that translate mechanical signals into biochemical signals resulting in modulation of signaling transduction pathways and change in cell physiology (Fig. 1). These adhesion proteins form adhesomes or adhesion nodes consisting of several proteins and anchor the cellular architecture of actomyosin to the outside microenvironment. Integrins are primary cell surface receptors that are part of adhesomes/focal adhesion nodes, mediate sensing of these non-soluble physical cues and transmit these signals to downstream effectors [2]. Integrins are transmembrane heterodimeric proteins that have two non-covalently bound α and β subunits (heterodimers) with large extracytoplasmic domains that detect the integrin-binding motif (arginine, glycine, aspartic acid—RGD) and a small intracytoplasmic domains that are attached to other proteins in focal adhesion nodes and cytoskeleton [81]. There are 18 α and 8 β integrin genes that form 24 heterodimers and multiple integrin heterodimers can gather in the adhesion nodes. These heterodimers of integrins have different specificity towards the mechanical cues resulting in differential cellular response as heterodimers with α5β1 determine the adhesion strength and are independent of receptor protein Tyr phosphatase-α (RPTPα), while αvβ3 are dependent on RPTPα signaling and determine the mechanotransduction [82, 83]. Also, the specificity of the integrin binding/heterodimers was found to be different in physiologically relevant 3D culture system compare to 2D culture as αv-integrins predominantly determines fate of stem cells and their differentiation in comparison to α5-integrins [11].
Cells regulate affinity of integrin dimers to recognize the binding motifs by protein kinase C signaling, thereby exerting some control over the outside-in signaling. Also, the clustering/spacing of integrins and focal adhesion nodes also determines downstream signaling, change in cell morphology, or even apoptosis in stem cells (low density of adhesion nodes causes cell death), highlighting the importance of spatial arrangement of these nodes [84–86]. These macromolecular tuning of integrins also determines the transmission strength of mechanotransduction signals [87]. On the cytoplasmic side, integrins are attached to proteins that tether them to cytoskeleton (e.g., talins), connect proteins in adhesion nodes serving as stabilizing bridges (e.g., Paxillin), or activate signal transduction pathways (e.g., FAK, ILK, Src). Interestingly, among several of integrin interacting proteins, few are capable of detecting variable mechanical signals including p130Cas, talin-vinculin system, and α-catenin [88–90]. These mechanosensitive proteins act as force sensor and are capable of controlling the duration and strength of downstream signaling activation and actin cytoskeleton remodeling. Apart from integrin/focal adhesion node-based mechanosensing, cells also utilize mechanosensitive ion channels to detect external physical cues and lead to diverse intracellular functions due to opening of these ion channels [91]. Several of these ion channels are activated by the tension developed on the surface of cell membrane and can be regulated by diverse physiological stimuli including extracellular osmotic changes but few of them (includes MEC-4/MEC-10, TRP, and Piezo family of channels) also require force sensing through focal adhesion nodes for their regulation and regulates cell behavior [92–94]. A list of membrane bound mechanosensors is provided in Table 2. The current understanding of mechanosensor ion channels that are linked with focal adhesion nodes indicate their role only during stem cell differentiation but importance of calcium ions flux on pluripotency/multipotency in stem cells and presence of multiple isoforms of these mechanosensor ion channels in stem cells suggest their possible role in stem cells renewal/viability.
The mechanical inputs from focal adhesion nodes are transmitted downstream via signaling transduction pathways. These signaling pathways regulate diverse cellular processes including metabolism/viability/proliferation (AKT, MAPK, JNK) and cell motility/spreading/polarity (Rho pathway and Hippo pathway) [111, 112]. Activation of tyrosine kinases with subsequent metabolic/viability pathway activation leads to post translational changes that sustains the metabolic needs/nutrient uptake in cells while also maintaining the viability. In embryonic stem cells, MAPK activation is essential to maintain pluripotency and cell proliferation [113], and it is also the central mechanotransduction pathway suggesting the congruence of mechanotransduction signaling and stem cell biology. MAPK signaling also cooperates with the AKT pathway to maintain the cellular needs of nutrients/higher glycolysis [114] in cells to sustain proliferation. Integrin signaling regulates both MAPK and AKT signaling, and mechanical cues provide the fine tuning of these pathways. Similarly, activation of guanine nucleotide exchange factors (GEFs) in the focal adhesion nodes leads to activation of GTPases in RhoA pathway (RhoA, Rac1, and others). RhoA and Rac1 take the inputs from adhesion nodes and directly regulate motility and cell protrusion or induce tension/stress fiber formation, thus modulating cellular architecture to adapt to mechanical cues. Activity of RhoA and Rac1 also determines the differentiation potential and lineage specification in stem cells [37, 115]. Inhibition of actomyosin regulators or Rho kinase has direct effect on viability of pluripotent stem cells, their proliferation and self-renewal for multiple passages [116, 117]. These results highlight that RhoA pathway which regulates the actin cytoskeleton tension in cells (non-muscle cells) has direct bearing on the stem cell biology. The connectivity of extracellular non-soluble (mechanical) cues and their translation through focal adhesion nodes results in response through either growth pathways (MAPK/AKT) or through change in cellular morphology using RhoA signaling. These pathways are critical in stem cells biology as they regulate pluripotency/multipotency and differentiation potential and are heavily regulated at multiple levels from both inside and outside the cells.
Placing Stem Cell Mechanobiology in Context
Considering the combinatorial nature of physiological cues presented to stem cells, particularly in response to injury, decision-making processes for stem cells to either self-renew, remain quiescent, migrate, or differentiate into one of the potential somatic cell type would necessarily involve complex signal integration and processing. Mechanical cues, for the most part, are persistent cues that stem cells receive for long periods in their lifetime and may not necessarily change in the case of acute injury distant from the niche. In such cases when mechanics surrounding stem cells remain static, even though new signals received by cells need to be responded to, do mechanical cues continue to matter? Is it possible that they act as permissive cues, allowing for certain phenotypes but only in the context of other non-mechanical cues. This contextual nature of mechanical signaling is important when ECM itself has altered mechanical properties. In aged individuals, for example, a different range of rigidity moduli and dis-repaired topography may not “permit” for certain stem cell behaviors that the correct physiological mechanical microenvironment would. These concerns exist also in wounded and scarred tissue, for example, in heart attack, where scarring dramatically and profoundly changes the mechanics of the microenvironment, mostly making it stiffer and less organized. Such changes in mechanics result in decreased capability for the tissue to regenerate or be repaired, increasing the chances of further damage, limiting functionality, and leading the tissue into a vicious cycle of disrepair. Therefore, it is necessary to both study mechanics in the context of other cues and, importantly, study paracrine and immune signaling in the context of mechanical cues. Mechanical cues are persistently present, and their correct dosage and stimulation are necessary for the correct and predicted behavior of stem cell-mediated tissue homeostasis.
The context of mechanics becomes even more important when mechanical cues get disrupted acutely, or cells experience different sets of mechanical environment during migration. These contexts can be present in a wound, remodeled tissue, as well as in cell encapsulated tissue engineered grafts when cells migrate from the graft to native tissue with potentially different mechanical microenvironment. Tools to study acute changes in mechanical properties of ECM are only now being developed, and soon they would be incorporated in reductive scientific experiments to investigate the role of dynamic mechanical microenvironment in influencing stem cell properties. Mechanics of the matrix are constant companions of stem cells, and their active signaling is essential to nearly all their behaviors. However, their role should be studied in a broader physiological context comprising of other signals that stem cells receive, process, and respond to.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Kshitiz, Kim DH, Beebe DJ, et al. Micro- and nanoengineering for stem cell biology: the promise with a caution. Trends Biotechnol. 2011;29(8):399–408.
Kshitiz, Park J, Kim P, et al. Control of stem cell fate and function by engineering physical microenvironments. Integr Biol. 2012;4(9):1008–18.
Dado D, Sagi M, Levenberg S, et al. Mechanical control of stem cell differentiation. Regen Med. 2012;7(1):101–16.
Moore SW, Roca-Cusachs P, Sheetz MP. Stretchy proteins on stretchy substrates: the important elements of integrin-mediated rigidity sensing. Dev Cell. 2010;19(2):194–206.
Kshitiz, Afzal J, Kim SY, et al. A nanotopography approach for studying the structure-function relationships of cells and tissues. Cell Adhes Migr. 2015;9(4):300–7.
Gupta K, Kim DH, Ellison D, et al. Lab-on-a-chip devices as an emerging platform for stem cell biology. Lab Chip. 2010;10(16):2019–31.
Ivanovska IL, Shin JW, Swift J, et al. Stem cell mechanobiology: diverse lessons from bone marrow. Trends Cell Biol. 2015;25(9):523–32.
Mesa KR, Rompolas P, Greco V. The dynamic duo: niche/stem cell interdependency. Stem Cell Reports. 2015;4(6):961–6.
Dalby MJ, Gadegaard N, Oreffo RO. Harnessing nanotopography and integrin-matrix interactions to influence stem cell fate. Nat Mater. 2014;13(6):558–69.
Humphrey JD, Dufresne ER, Schwartz MA. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol. 2014;15(12):802–12.
Huebsch N, Arany PR, Mao AS, et al. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat Mater. 2010;9(6):518–26.
Kshitiz, Hubbi ME, Ahn EH, et al. Matrix rigidity controls endothelial differentiation and morphogenesis of cardiac precursors. Sci Signal. 2012;5(227):ra41. This study suggests that extracellular mechanical forces can be sensed by progenitor cells and transmitted by a mechanosensor: p190RhoGAP which temporally coordinates various phenotypic outcomes including proliferation, differentiation, and morphogenesis.
Chaudhuri O, Gu L, Klumpers D, et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat Mater. 2015.
Kuo YC, Chang TH, Hsu WT, et al. Oscillatory shear stress mediates directional reorganization of actin cytoskeleton and alters differentiation propensity of mesenchymal stem cells. Stem Cells. 2015;33(2):429–42.
Kim KM, Choi YJ, Hwang JH, et al. Shear stress induced by an interstitial level of slow flow increases the osteogenic differentiation of mesenchymal stem cells through TAZ activation. PLoS One. 2014;9(3), e92427.
Das RK, Gocheva V, Hammink R et al. Stress-stiffening-mediated stem-cell commitment switch in soft responsive hydrogels. Nat Mater. 2015. This report highlights that onset of stiffening of 3D matrices encapsulating mesenchymal stem cells is itself sufficient to alter the differentiated fate of the cells.
Earls JK, Jin S, Ye K. Mechanobiology of human pluripotent stem cells. Tissue Eng B Rev. 2013;19(5):420–30.
Stolberg S, McCloskey KE. Can shear stress direct stem cell fate? Biotechnol Prog. 2009;25(1):10–9.
Gilbert PM, Havenstrite KL, Magnusson KE, et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329(5995):1078–81.
Fu J, Wang YK, Yang MT, et al. Mechanical regulation of cell function with geometrically modulated elastomeric substrates. Nat Methods. 2010;7(9):733–6.
Tse JR, Engler AJ. Stiffness gradients mimicking in vivo tissue variation regulate mesenchymal stem cell fate. PLoS One. 2011;6(1):e15978. This study suggests that gradients of rigidity of matrix itself could serve as an instructive cue for stem cells to regulate their fate.
Du J, Chen X, Liang X, et al. Integrin activation and internalization on soft ECM as a mechanism of induction of stem cell differentiation by ECM elasticity. Proc Natl Acad Sci U S A. 2011;108(23):9466–71.
Wen JH, Vincent LG, Fuhrmann A, et al. Interplay of matrix stiffness and protein tethering in stem cell differentiation. Nat Mater. 2014;13(10):979–87.
Ahn EH, Kim Y, Kshitiz, et al. Spatial control of adult stem cell fate using nanotopographic cues. Biomaterials. 2014;35(8):2401–10.
Guilak F, Cohen DM, Estes BT, et al. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell. 2009;5(1):17–26.
Kolind K, Leong KW, Besenbacher F, et al. Guidance of stem cell fate on 2D patterned surfaces. Biomaterials. 2012;33(28):6626–33.
Lapointe VL, Fernandes AT, Bell NC, et al. Nanoscale topography and chemistry affect embryonic stem cell self-renewal and early differentiation. Adv Healthc Mater. 2013;2(12):1644–50.
Lee J, Abdeen AA, Zhang D, et al. Directing stem cell fate on hydrogel substrates by controlling cell geometry, matrix mechanics and adhesion ligand composition. Biomaterials. 2013;34(33):8140–8.
Oh S, Brammer KS, Li YS, et al. Stem cell fate dictated solely by altered nanotube dimension. Proc Natl Acad Sci U S A. 2009;106(7):2130–5.
Connelly JT, Gautrot JE, Trappmann B, et al. Actin and serum response factor transduce physical cues from the microenvironment to regulate epidermal stem cell fate decisions. Nat Cell Biol. 2010;12(7):711–8.
Abdeen AA, Weiss JB, Lee J, et al. Matrix composition and mechanics direct proangiogenic signaling from mesenchymal stem cells. Tissue Eng A. 2014;20(19–20):2737–45.
Murphy CM, Matsiko A, Haugh MG, et al. Mesenchymal stem cell fate is regulated by the composition and mechanical properties of collagen-glycosaminoglycan scaffolds. J Mech Behav Biomed Mater. 2012;11:53–62.
Faia-Torres AB, Guimond-Lischer S, Rottmar M, et al. Differential regulation of osteogenic differentiation of stem cells on surface roughness gradients. Biomaterials. 2014;35(33):9023–32.
Sarkar D, Ankrum JA, Teo GS, et al. Cellular and extracellular programming of cell fate through engineered intracrine-, paracrine-, and endocrine-like mechanisms. Biomaterials. 2011;32(11):3053–61.
Vallier L, Alexander M, Pedersen RA. Activin/nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J Cell Sci. 2005;118(Pt 19):4495–509.
Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417(6884):39–44.
Gao L, McBeath R, Chen CS. Stem cell shape regulates a chondrogenic versus myogenic fate through Rac1 and N-cadherin. Stem Cells. 2010;28(3):564–72.
Zhang J, Woodhead GJ, Swaminathan SK, et al. Cortical neural precursors inhibit their own differentiation via N-cadherin maintenance of beta-catenin signaling. Dev Cell. 2010;18(3):472–9.
van Es JH, van Gijn ME, Riccio O, et al. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435(7044):959–63.
Morrison SJ, Perez SE, Qiao Z, et al. Transient Notch activation initiates an irreversible switch from neurogenesis to gliogenesis by neural crest stem cells. Cell. 2000;101(5):499–510.
Salgado AJ, Sousa JC, Costa BM, et al. Mesenchymal stem cells secretome as a modulator of the neurogenic niche: basic insights and therapeutic opportunities. Front Cell Neurosci. 2015;9:249.
Sart S, Liu Y, Ma T, et al. Microenvironment regulation of pluripotent stem cell-derived neural progenitor aggregates by human mesenchymal stem cell secretome. Tissue Eng A. 2014;20(19–20):2666–79.
Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324(5935):1673–7.
Liu S, Ginestier C, Ou SJ, et al. Breast cancer stem cells are regulated by mesenchymal stem cells through cytokine networks. Cancer Res. 2011;71(2):614–24.
Chadwick K, Wang L, Li L, et al. Cytokines and BMP-4 promote hematopoietic differentiation of human embryonic stem cells. Blood. 2003;102(3):906–15.
Menendez L, Yatskievych TA, Antin PB, et al. Wnt signaling and a Smad pathway blockade direct the differentiation of human pluripotent stem cells to multipotent neural crest cells. Proc Natl Acad Sci U S A. 2011;108(48):19240–5.
Ling L, Nurcombe V, Cool SM. Wnt signaling controls the fate of mesenchymal stem cells. Gene. 2009;433(1–2):1–7.
Katoh M, Katoh M. Cross-talk of WNT and FGF signaling pathways at GSK3beta to regulate beta-catenin and SNAIL signaling cascades. Cancer Biol Ther. 2006;5(9):1059–64.
Mohyeldin A, Garzon-Muvdi T, Quinones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010;7(2):150–61.
Holmstrom KM, Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat Rev Mol Cell Biol. 2014;15(6):411–21.
Bernstein E, Kim SY, Carmell MA, et al. Dicer is essential for mouse development. Nat Genet. 2003;35(3):215–7.
Kanellopoulou C, Muljo SA, Kung AL, et al. Dicer-deficient mouse embryonic stem cells are defective in differentiation and centromeric silencing. Genes Dev. 2005;19(4):489–501.
Pauli A, Rinn JL, Schier AF. Non-coding RNAs as regulators of embryogenesis. Nat Rev Genet. 2011;12(2):136–49.
Hou P, Li Y, Zhang X, et al. Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science. 2013;341(6146):651–4.
Engler AJ, Sen S, Sweeney HL, et al. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–89.
Yahalom-Ronen Y, Rajchman D, Sarig R et al. Reduced matrix rigidity promotes neonatal cardiomyocyte dedifferentiation, proliferation and clonal expansion. Elife. 2015;4.
Majkut S, Idema T, Swift J, et al. Heart-specific stiffening in early embryos parallels matrix and myosin expression to optimize beating. Curr Biol. 2013;23(23):2434–9.
Shkumatov A, Baek K, Kong H. Matrix rigidity-modulated cardiovascular organoid formation from embryoid bodies. PLoS One. 2014;9(4), e94764.
Arshi A, Nakashima Y, Nakano H et al. Rigid microenvironments promote cardiac differentiation of mouse and human embryonic stem cells. Sci Technol Adv Mater. 2013;14(2).
Sun Y, Villa-Diaz LG, Lam RH, et al. Mechanics regulates fate decisions of human embryonic stem cells. PLoS One. 2012;7(5), e37178.
Raab M, Shin JW, Discher DE. Matrix elasticity in vitro controls muscle stem cell fate in vivo. Stem Cell Res Ther. 2010;1(5):38.
Hariharan N, Sussman MA. Cardiac aging—getting to the stem of the problem. J Mol Cell Cardiol. 2015;83:32–6.
Bae D, Moon SH, Park BG, et al. Nanotopographical control for maintaining undifferentiated human embryonic stem cell colonies in feeder free conditions. Biomaterials. 2014;35(3):916–28.
Lim SH, Liu XY, Song H, et al. The effect of nanofiber-guided cell alignment on the preferential differentiation of neural stem cells. Biomaterials. 2010;31(34):9031–9.
Kim DH, Kshitiz, Smith RR, et al. Nanopatterned cardiac cell patches promote stem cell niche formation and myocardial regeneration. Integr Biol. 2012;4(9):1019–33.
Kshitiz, Afzal J, Kim DH, et al. Concise review: Mechanotransduction via p190RhoGAP regulates a switch between cardiomyogenic and endothelial lineages in adult cardiac progenitors. Stem Cells. 2014;32(8):1999–2007. Our review highlighting that a single mechanosensor could act as a switch regulating different outcomes in cardiac progenitors, and that this mechanosensor can be tuned by extracellular mechanical cues.
Kim ES, Ahn EH, Chung E, et al. Recent advances in nanobiotechnology and high-throughput molecular techniques for systems biomedicine. Mol Cells. 2013;36(6):477–84.
Song S, Kim EJ, Bahney CS, et al. The synergistic effect of micro-topography and biochemical culture environment to promote angiogenesis and osteogenic differentiation of human mesenchymal stem cells. Acta Biomater. 2015;18:100–11.
Ogino Y, Liang R, Mendonca DB, et al. RhoA-mediated functions in C3H10T1/2 osteoprogenitors are substrate topography dependent. J Cell Physiol. 2016;231(3):568–75.
Yang K, Park E, Lee JS, et al. Biodegradable nanotopography combined with neurotrophic signals enhances contact guidance and neuronal differentiation of human neural stem cells. Macromol Biosci. 2015;15(10):1348–56.
Abagnale G, Steger M, Nguyen VH, et al. Surface topography enhances differentiation of mesenchymal stem cells towards osteogenic and adipogenic lineages. Biomaterials. 2015;61:316–26.
Abboud FM, Huston JH. The effects of aging and degenerative vascular disease on the measurement of arterial rigidity in man. J Clin Invest. 1961;40:933–9.
Horn MA and Trafford AW. Aging and the cardiac collagen matrix: novel mediators of fibrotic remodeling. J Mol Cell Cardiol. 2015.
Lynch K, Pei M. Age associated communication between cells and matrix: a potential impact on stem cell-based tissue regeneration strategies. Organogenesis. 2014;10(3):289–98.
Clarke SA, Richardson WJ, Holmes JW. Modifying the mechanics of healing infarcts: is better the enemy of good? J Mol Cell Cardiol. 2015.
Li AH, Liu PP, Villarreal FJ, et al. Dynamic changes in myocardial matrix and relevance to disease: translational perspectives. Circ Res. 2014;114(5):916–27.
Gunes SI, Jana SC. Shape memory polymers and their nanocomposites: a review of science and technology of new multifunctional materials. J Nanosci Nanotechnol. 2008;8(4):1616–37.
Trappmann B, Gautrot JE, Connelly JT, et al. Extracellular-matrix tethering regulates stem-cell fate. Nat Mater. 2012;11(7):642–9.
Chowdhury F, Li Y, Poh YC, et al. Soft substrates promote homogeneous self-renewal of embryonic stem cells via downregulating cell-matrix tractions. PLoS One. 2010;5(12), e15655.
Chowdhury F, Na S, Li D, et al. Material properties of the cell dictate stress-induced spreading and differentiation in embryonic stem cells. Nat Mater. 2010;9(1):82–8.
Takada Y, Ye X, Simon S. The integrins. Genome Biol. 2007;8(5):215.
Jiang G, Huang AH, Cai Y, et al. Rigidity sensing at the leading edge through alphavbeta3 integrins and RPTPalpha. Biophys J. 2006;90(5):1804–9.
Roca-Cusachs P, Gauthier NC, Del Rio A, et al. Clustering of alpha(5)beta(1) integrins determines adhesion strength whereas alpha(v)beta(3) and talin enable mechanotransduction. Proc Natl Acad Sci U S A. 2009;106(38):16245–50.
Cavalcanti-Adam EA, Volberg T, Micoulet A, et al. Cell spreading and focal adhesion dynamics are regulated by spacing of integrin ligands. Biophys J. 2007;92(8):2964–74.
Cavalcanti-Adam EA, Aydin D, Hirschfeld-Warneken VC, et al. Cell adhesion and response to synthetic nanopatterned environments by steering receptor clustering and spatial location. HFSP J. 2008;2(5):276–85.
Dalby MJ, Gadegaard N, Tare R, et al. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat Mater. 2007;6(12):997–1003.
Yim EK, Darling EM, Kulangara K, et al. Nanotopography-induced changes in focal adhesions, cytoskeletal organization, and mechanical properties of human mesenchymal stem cells. Biomaterials. 2010;31(6):1299–306.
Sawada Y, Tamada M, Dubin-Thaler BJ, et al. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell. 2006;127(5):1015–26.
del Rio A, Perez-Jimenez R, Liu R, et al. Stretching single talin rod molecules activates vinculin binding. Science. 2009;323(5914):638–41.
Yonemura S, Wada Y, Watanabe T, et al. alpha-Catenin as a tension transducer that induces adherens junction development. Nat Cell Biol. 2010;12(6):533–42.
Arnadottir J, Chalfie M. Eukaryotic mechanosensitive channels. Annu Rev Biophys. 2010;39:111–37.
Pathak MM, Nourse JL, Tran T, et al. Stretch-activated ion channel Piezo1 directs lineage choice in human neural stem cells. Proc Natl Acad Sci U S A. 2014;111(45):16148–53.
Martinac B. The ion channels to cytoskeleton connection as potential mechanism of mechanosensitivity. Biochim Biophys Acta. 2014;1838(2):682–91.
Hayakawa K, Tatsumi H, Sokabe M. Actin stress fibers transmit and focus force to activate mechanosensitive channels. J Cell Sci. 2008;121(Pt 4):496–503.
Lund AW, Stegemann JP, Plopper GE. Mesenchymal stem cells sense three dimensional type I collagen through discoidin domain receptor 1. Open Stem Cell J. 2009;1:40–53.
Suh HN, Han HJ. Collagen I regulates the self-renewal of mouse embryonic stem cells through alpha2beta1 integrin- and DDR1-dependent Bmi-1. J Cell Physiol. 2011;226(12):3422–32.
Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15(12):786–801.
Chang C, Goel HL, Gao H, et al. A laminin 511 matrix is regulated by TAZ and functions as the ligand for the alpha6Bbeta1 integrin to sustain breast cancer stem cells. Genes Dev. 2015;29(1):1–6.
Pisconti A, Cornelison DD, Olguin HC, et al. Syndecan-3 and Notch cooperate in regulating adult myogenesis. J Cell Biol. 2010;190(3):427–41.
Selleri C, Ragno P, Ricci P, et al. The metastasis-associated 67-kDa laminin receptor is involved in G-CSF-induced hematopoietic stem cell mobilization. Blood. 2006;108(7):2476–84.
Mederos y Schnitzler M, Storch U, Gudermann T. AT1 receptors as mechanosensors. Curr Opin Pharmacol. 2011;11(2):112–6.
Liu YS, Liu YA, Huang CJ, et al. Mechanosensitive TRPM7 mediates shear stress and modulates osteogenic differentiation of mesenchymal stromal cells through Osterix pathway. Sci Rep. 2015;5:16522.
Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7(9):678–89.
Dalagiorgou G, Basdra EK, Papavassiliou AG. Polycystin-1: function as a mechanosensor. Int J Biochem Cell Biol. 2010;42(10):1610–3.
Kim ST, Takeuchi K, Sun ZY, et al. The alphabeta T cell receptor is an anisotropic mechanosensor. J Biol Chem. 2009;284(45):31028–37.
Drummond HA, Jernigan NL, Grifoni SC. Sensing tension: epithelial sodium channel/acid-sensing ion channel proteins in cardiovascular homeostasis. Hypertension. 2008;51(5):1265–71.
Bao L, Sachs F, Dahl G. Connexins are mechanosensitive. Am J Physiol Cell Physiol. 2004;287(5):C1389–95.
Lee W, Leddy HA, Chen Y, et al. Synergy between Piezo1 and Piezo2 channels confers high-strain mechanosensitivity to articular cartilage. Proc Natl Acad Sci U S A. 2014;111(47):E5114–22.
Coste B, Mathur J, Schmidt M, et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330(6000):55–60.
Chen Y, Bharill S, Isacoff EY, et al. Subunit composition of a DEG/ENaC mechanosensory channel of Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2015;112(37):11690–5.
Legate KR, Wickstrom SA, Fassler R. Genetic and cell biological analysis of integrin outside-in signaling. Genes Dev. 2009;23(4):397–418.
Halder G, Dupont S, Piccolo S. Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat Rev Mol Cell Biol. 2012;13(9):591–600.
Li J, Wang G, Wang C, et al. MEK/ERK signaling contributes to the maintenance of human embryonic stem cell self-renewal. Differentiation. 2007;75(4):299–307.
Robey RB, Hay N. Is Akt the “Warburg kinase”?-Akt-energy metabolism interactions and oncogenesis. Semin Cancer Biol. 2009;19(1):25–31.
Sordella R, Jiang W, Chen GC, et al. Modulation of Rho GTPase signaling regulates a switch between adipogenesis and myogenesis. Cell. 2003;113(2):147–58.
Walker A, Su H, Conti MA, et al. Non-muscle myosin II regulates survival threshold of pluripotent stem cells. Nat Commun. 2010;1:71.
Watanabe K, Ueno M, Kamiya D, et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25(6):681–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Kshitiz has a patent WO2013151755 A1 licensed to Nanosurface Biomedical, Inc. and a patent WO2014144219 A1 licensed to Nanosurface Biomedical, Inc.
Junaid Afzal, Hao Chang, Ruchi Goyal, and Andre Levchenko declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Stem Cells and Nanotechnologies
Rights and permissions
About this article
Cite this article
Kshitiz, Afzal, J., Chang, H. et al. Mechanics of Microenvironment as Instructive Cues Guiding Stem Cell Behavior. Curr Stem Cell Rep 2, 62–72 (2016). https://doi.org/10.1007/s40778-016-0033-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40778-016-0033-9