Abstract
We investigate the context of discovery of two significant achievements of twentieth century biochemistry: the chemiosmotic mechanism of oxidative phosphorylation (proposed in 1961 by Peter Mitchell) and the dark reaction of photosynthesis (elucidated from 1946 to 1954 by Melvin Calvin and Andrew A. Benson). The pursuit of these problems involved discovery strategies such as the transfer, recombination and reversal of previous causal and mechanistic knowledge in biochemistry. We study the operation and scope of these strategies by careful historical analysis, reaching a number of systematic conclusions: (1) even basic strategies can illuminate “hard cases” of scientific discovery that go far beyond simple extrapolation or analogy; (2) the causal–mechanistic approach to discovery permits a middle course between the extremes of a completely substrate-neutral and a completely domain-specific view of scientific discovery; (3) the existing literature on mechanism discovery underemphasizes the role of combinatorial approaches in defining and exploring search spaces of possible problem solutions; (4) there is a subtle interplay between a fine-grained mechanistic and a more coarse-grained causal level of analysis, and both are needed to make discovery processes intelligible.







Similar content being viewed by others
Notes
For conceptual analysis, see particularly Simon (1973), the essays in Nickles (1980b), and Hoyningen-Huene (1987, 2006). For early historical work, see the case studies in Nickles (1980a), Darden (1991), Bechtel and Richardson (1993) and Schaffner (1994). More recently, scientific discovery was reinvigorated by the volumes by Bechtel (2005), Darden (2006), Schickore and Steinle (2006), Meheus and Nickles (2009) and Craver and Darden (2013). See also references cited therein.
Our focus on causal–mechanistic reasoning strategies is not meant to be exclusionary: We believe that our approach is one of many useful points of view. First, we deal with our case studies at a much less fine-grained resolution than analyses of laboratory notebooks (see especially Holmes et al. 2003; Holmes 2004). Like Holmes, we believe that multiple scales of temporal “graininess” can each reveal important aspects of the context of discovery (2004, Chapter 2; 2009, pp. 77–78). Second, our study is not a contribution to cognitive science because we do not analyse the process of hypothesis generation in terms of the immediate mental operations performed by scientists (cf. Holmes 1991; Holyoak and Thagard 1996; Nersessian 2008, and see references therein). For the same reason, moreover, we do not hold that the strategies we describe could be implemented computationally so as to automate scientific discovery: we do not know if this will ever be possible, but at this stage of the project it certainly is not. Finally, we do not use Rheinberger’s (1997) “experimental systems” approach because we have a different focus: like Weber (2005, Chapter 5) we take a methodological analysis of hypothesis generation to be a necessary complement to the study of the capacities of experimental systems.
We do not commit to any particular philosophical account of causation. We expect that our epistemological conclusions will be compatible with whatever the best metaphysical analysis of causation turns out to be—be it a probabilistic theory, a counterfactual theory, a process theory, or something else.
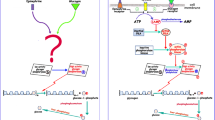
We present a schema of the mechanism accepted today as a guide for readers who are unfamiliar with the biochemical details. Mitchell’s original proposal differed from this schema in several respects. For instance, his proposal imagined the orientation of the membrane transport systems (components 2 and 3) to be the reverse of what is actually the case. Moreover, Mitchell included a fourth component: an ion exchange system which would allow \(\hbox {H}^{+}/\hbox {K}^{+}\)-exchange, reducing the pH differential and replacing it with a potassium membrane potential (also driving \(\hbox {H}^{+}\) flow). Finally, Mitchell’s hypotheses about the mechanisms by which these components operate differed significantly from what is accepted today.
For a contemporary overview of some of these hypotheses, see Danielli (1954).
It is telling that Mitchell and Moyle argued for their theory of membrane transport by highlighting not its originality but its orthodoxy (Mitchell and Moyle 1958, p. 373). Although the Popperians are no longer on the scene, the prejudice still prevails that scientists generally aim to propose revolutionary hypotheses (for a discussion and critique of this position, see Chalmers 1973). But while iconoclasm is certainly a desirable long-term outcome, conservatism seems to be more highly prized in everyday hypothesis generation (trying to explain oxidative phosphorylation by analogy to glycolysis, or membrane transport in terms of enzyme reactions). We will have more such instances in our second case study in Sect. 4.
Robertson distinguishes between charge separation as a process occurring in the membrane-bound cytochrome system and secretion as one result of charge separation.
See Robinson (1997) for a discussion of the history of the redox pump hypothesis on pp. 113–116.
See Joseph D. Robinson’s invaluable history of membrane transport, especially chapters 3–8, for an in-depth discussion of these developments (Robinson 1997).
In recognition of the discovery, Skou received the Nobel Prize in Chemistry in 1997, together with Paul D. Boyer and John E. Walker.
Prebble (2000) notes that Mitchell “came up with mechanisms in large numbers” and “was allergic to systems that depended on processes which at the time could not be defined precisely at the molecular level” (p. 330). He defends Mitchell’s penchant for inventing mechanisms with the suggestion that a few successes justify “so many apparently fruitless mechanisms”. We would argue that no defense is needed: scientific discovery relies on the proposal of appropriate candidate mechanisms. Moreover, we are not sure to what extent Mitchell’s insistence on molecular detail was unusual, since at least Robertson (as discussed above) also formulated hypotheses largely at the mechanistic level. Prebble mentions that many further ideas for mechanisms exist in Mitchell’s unpublished documents, and it will be worthwhile to investigate these in the light of a causal–mechanistic understanding of discovery.
A pointed comment on this fact as well as on Calvin’s failure to acknowledge Benson’s contribution in public ever since is provided by Fuller (1999), pp. 9–10.
This is not meant to suggest that the radiotracer techniques were unimportant. However, an appropriate treatment would double the paper’s length.
The abbreviation [\(\hbox {C}_2\)] denotes that this compound contains two carbon atoms, usually as a backbone chain. Within a compound the carbons are given numbers according to their position in the chain.
This compound is known today as “ribulose bisphosphate”; in order to be consistent with the historical sources, the older name has been adopted for the paper.
References
Alberts, B., Johnson, A., Lewis, J., Raff, M., Roberts, K., & Walter, P. (2002). Molecular biology of the cell. New York: Garland Science.
Allchin, D. (1992). How do you falsify a question? crucial tests versus crucial demonstrations. In PSA: Proceedings of the Biennial Meeting of the Philosophy of Science Association (pp. 74–88).
Allchin, D. (1994). The super bowl and the Ox-Phos Controversy: “Winner-take-all” competition in philosophy of science. In PSA: Proceedings of the Biennial Meeting of the Philosophy of Science Association (pp. 22–33).
Allchin, D. (1997). A 20th century phlogiston: Constructing error and differentiating domains. Perspectives on Science, 5(1), 81–127.
Arabatzis, T. (2006). On the inextricability of the context of discovery and the context of justification. In J. Schickore & F. Steinle (Eds.), Revisiting discovery and justification: Historical and philosophical perspectives on the context distinction (pp. 215–230). Dordrecht: Springer.
Bassham, J. A., Benson, A. A., Kay, L. D., Harris, A. Z., Wilson, A. T., & Calvin, M. (1954). The path of carbon in photosynthesis. XXI. The cyclic regeneration of carbon dioxide acceptor. Journal of the American Chemical Society, 76, 1760–1770.
Bechtel, W. (2005). Discovering cell mechanisms: The creation of modern cell biology. Cambridge: Cambridge University Press.
Bechtel, W., & Richardson, R. C. (1993). Discovering Complexity. Decomposition and Localization as Strategies in Scientific Research. Princeton: Princeton University Press.
Benson, A. A. (1951). Identification of ribulose in \(^{14}{\rm CO}_2\) photosynthesis products. Journal of the American Chemical Society, 73, 2971–2972.
Benson, A. A., Bassham, J. A., & Calvin, M. (1951). Sedoheptulose in photosynthesis by plants. Journal of the American Chemical Society, 73, 2970.
Benson, A. A., Bassham, J. A., Calvin, M., Hall, A. G., Hirsch, H. E., Kawaguchi, S., et al. (1952). The path of carbon in photosynthesis. XV. Ribulose and sedoheptulose. Journal of Biological Chemistry, 196, 703–716.
Benson, A. A., & Calvin, M. (1947). The dark reductions of photosynthesis. Science, 105, 648–649.
Benson, A. A., & Calvin, M. (1950). Carbon dioxide fixation by green plants. Annual Review of Plant Physiology, 1, 25–42.
Calvin, M., & Benson, A. A. (1948). The path of carbon in photosynthesis. Science, 107, 476–480.
Calvin, M., & Massini, P. (1952). The path of carbon in photosynthesis. XX: The steady state. Experientia, 8, 445–457.
Chalmers, A. (1973). On learning from our mistakes. British Journal for the Philosophy of Science, 24(2), 164–173.
Craver, C. F., & Darden, L. (2013). In search of mechanisms: Discoveries across the life sciences. Chicago: University of Chicago Press.
Danielli, J. F. (1954). Morphological and molecular aspects of active transport. In R. Brown & J. F. Danielli (Eds.), Active transport and secretion, vol. 8 of Symposia of the Society for Experimental Biology (pp. 502–516). Cambridge University Press.
Darden, L. (1991). Theory change in science: Strategies from Mendelian genetics. New York: Oxford University Press.
Darden, L. (2006). Reasoning in biological discoveries. New York: Cambridge University Press.
Evans, M. C., Buchanan, R. B., & Arnon, D. I. (1966). A new ferredoxin-dependent carbon reduction cycle in a photosynthetic bacterium. Proceedings of the National Academy of Sciences (USA), 55, 928–934.
Fuller, R. C. (1999). Forty years of microbial photosynthesis research: Where it came from and what it led to. Photosynthesis Research, 62, 1–29.
Goodwin, T. W., & Lindberg, O. (Eds.). (1961). Biological structure and function. London: Academic Press.
Hatch, M. D. (1992). I can’t believe my luck. Photosynthesis Research, 33, 1–14.
Hatch, M. D. (2002). \({\text{ C}}_4\) photosynthesis: Discovery and resolution. Photosynthesis Research, 73, 251–256.
Hatch, M. D., & Slack, C. R. (1966). Photosynthesis in sugar cane leaves: A new carboxylation reaction and the pathway of sugar formation. Biochemical Journal, 101, 103–111.
Hatch, M. D., Slack, C. R., & Johnson, H. S. (1967). Further studies on a new pathway of photosynthetic \({\text{ CO}}_2\) fixation in sugarcane and its occurrence in other plant species. Biochemical Journal, 102, 417–422.
Holmes, F. L. (1991). Hans Krebs: The formation of a scientific life. New York: Oxford Pniversity Press.
Holmes, F. L. (2004). Investigative pathways: Patterns and stages in the careers of experimental scientists. New Haven: Yale University Press.
Holmes, F. L. (2009). Experimental systems, investigative pathways, and the nature of discovery. In J. Meheus & T. Nickles (Eds.), Models of discovery and creativity (pp. 65–79). Dordrecht: Springer.
Holmes, F. L., Renn, J., & Rheinberger, H.-J. (2003). Reworking the bench: Research notebooks in the history of science. Berlin: Springer.
Holyoak, K. J., & Thagard, P. (1996). Mental leaps: Analogy in creative thought. Cambridge: MIT press.
Hoyningen-Huene, P. (1987). On the varieties of the distinction between the context of discovery and the context of justification. Studies in History and Philosophy of Science, 18, 501–515.
Hoyningen-Huene, P. (2006). Context of discovery versus context of justification and Thomas Kuhn. In J. Schickore & F. Steinle (Eds.), Revisiting discovery and justification: Historical and philosophical perspectives on the context distinction (pp. 119–131). Dordrecht: Springer.
Keilin, D., & Hartree, E. F. (1939). Cytochrome and cytochrome oxidase. Proceedings of the Royal Society of London, Series B, 127, 167–191.
Kleinzeller, A., & Kotyk, A. (Eds.). (1961). Membrane transport and metabolism. New York: Academic Press.
Kortschak, H. P., Hartt, C. E., & Burr, G. O. (1965). Carbon dioxide fixation in sugarcane leaves. Plant Physiology, 40, 209–213.
Krebs, H. (1974). The discovery of carbon dioxide fixation in mammalian tissues. Molecular and Cellular Biochemistry, 5, 79–94.
Krebs, H., & Henseleit, K. (1932). Untersuchungen ueber die Harnstoffbildung im Tierkoerper. Hoppe-Seyler’s Zeitschrift fuer physiologische Chemie, 210, 33–66.
Krebs, H., & Johnson, W. A. (1937). Metabolism of ketonic acids in animal tissues. Biochemical Journal, 31, 645–660.
Lehninger, A. (1961). Components of the energy-coupling mechanism and mitochondrial structure. In T. W. Goodwin & O. Lindberg (Eds.), Biological structure and function, vol. 2 (pp. 31–51). New York: Academic.
Lehninger, A., Wadkins, C., Cooper, C., Devlin, T., & Gamble, J, Jr. (1958). Oxidative phosphorylation. Science, 128(3322), 450.
Levy, A., & Bechtel, W. (2013). Abstraction and the organization of mechanisms. Philosophy of Science, 80(2), 241–261.
Lipton, P. (2004). Inference to the best explanation. London: Routledge.
Machamer, P., Darden, L., & Craver, C. F. (2000). Thinking about mechanisms. Philosophy of Science, 67(1), 1–25.
Meheus, J., & Nickles, T. (2009). Models of discovery and creativity. Dordrecht: Springer.
Mitchell, P. (1949). The osmotic barrier in bacteria. In A. A. Miles & N. W. Pirie (Eds.), The nature of the bacterial surface. Oxford: Blackwell.
Mitchell, P. (1954). Transport of phosphate across the osmotic barrier of micrococcus pyogenes: Specificity and kinetics. Journal of General Microbiology, 11, 73–82.
Mitchell, P. (1957). A general theory of membrane transport from studies of bacteria. Nature, 180(4577), 134–136.
Mitchell, P. (1958). Membrane penetration and the therapeutic value of chemicals. In S. T. Cowan & E. Rowath (Eds.), The Strategy of Chemotherapy, vol. 8 of Symposium of the Society for General Microbiology, (pp. 94–103). Cambridge University Press.
Mitchell, P. (1961a). Approaches to the analysis of specific membrane transport. In T. W. Goodwin & O. Lindberg (Eds.), Biological structure and function, vol. 2 (pp. 581–599). New York: Academic.
Mitchell, P. (1961b). Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biochemical Journal, 79(3), 23P–24P.
Mitchell, P. (1961c). Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature, 191(4784), 144–148.
Mitchell, P., & Moyle, J. (1956a). Liberation and osmotic properties of the protoplasts of Micrococcus lysodeikticus and Sarcina lutea. Journal of General Microbiology, 15(3), 512–520.
Mitchell, P., & Moyle, J. (1956b). Osmotic function and structure in bacteria. In E. T. C. Spooner & B. A. D. Stocker (Eds.), Bacterial Anatomy, vol. 6 of Symposium of the Society for General Microbiology, (pp. 150–180). Cambridge University Press.
Mitchell, P., & Moyle, J. (1956c). Permeation mechanisms in bacterial membranes. Discussions of the Faraday Society, 21, 258–265.
Mitchell, P., & Moyle, J. (1956d). The cytochrome system in the plasma membrane of Staphylococcus aureus. Biochemical Journal, 64(1), 19P.
Mitchell, P., & Moyle, J. (1957). Autolytic release and osmotic properties of ‘Protoplasts’ from Staphylococcus aureus. Journal of General Microbiology, 16, 184–194.
Mitchell, P., & Moyle, J. (1958). Group-translocation: a consequence of enzyme-catalysed group-transfer. Nature, 182, 372–373.
Mitchell, P., & Moyle, J. (1959). Permeability of the envelopes of Staphylococcus aureus to some salts, amino acids, and non-electrolytes. Journal of General Microbiology, 20, 434–441.
Moses, V., & Moses, S. (2000). The Calvin Lab: Bio-Organic Chemistry Group at the University of California, Berkeley, 1945–1963. An oral history conducted 1995–1997, vol. 1. Regional Oral History Office, the Bancroft Library, University of California, Berkeley.
Nersessian, N. (2008). Creating scientific concepts. Cambridge: The MIT Press.
Nickelsen, K. (2009). The construction of a scientific model: Otto Warburg and the building block strategy. Studies in History and Philosophy of Biological and Biomedical Sciences, 40(2), 73–86.
Nickelsen, K. (2012). The path of carbon in photosynthesis: How to discover a biochemical pathway. Ambix, 59, 266–293.
Nickelsen, K. (2015). Explaining photosynthesis: Models of biochemical mechanisms. (1840–1960). Dordrecht: Springer.
Nickelsen, K., & Graßhoff, G. (2008). Concepts from the bench: Krebs and the urea cycle. In G. Hon, J. Schickore, & F. Steinle (Eds.), Going amiss in experimental research (pp. 91–117). Dordrecht: Springer.
Nickles, T. (1980a). Scientific discovery: Case studies, vol. 2. Dordrecht: Springer.
Nickles, T. (1980b). Scientific discovery, logic, and rationality, vol. 1. Dordrecht: Springer.
Popper, K. R. (2002). The logic of scientific discovery. London: Routledge.
Prebble, J. (1996). Successful theory development in biology: A consideration of the theories of oxidative phosphorylation proposed by Davies and Krebs, Williams and Mitchell. Bioscience Reports, 16(3), 207–215.
Prebble, J. (2000). The lasting value of Mitchell’s mechanisms. Nature, 404(6776), 330–330.
Prebble, J. (2001). The philosophical origins of Mitchell’s chemiosmotic concepts. Journal of the History of Biology, 34(3), 433–460.
Prebble, J., & Weber, B. (2003). Wandering in the gardens of the mind: Peter Mitchell and the making of Glynn. Oxford: Oxford University Press.
Prebble, J. N. (2010). The discovery of oxidative phosphorylation: A conceptual off-shoot from the study of glycolysis. Studies in History and Philosophy of Biological and Biomedical Sciences, 41(3), 253–262.
Quayle, J. R., Fuller, R. C., Benson, A. A., & Calvin, M. (1954). Enzymatic carboxylation of ribulose diphosphate. Journal of the American Chemical Society, 76, 3610–3612.
Racker, E., & Stoeckenius, W. (1974). Reconstitution of purple membrane vesicles catalyzing light-driven proton uptake and adenosine triphosphate formation. Journal of Biological Chemistry, 249(2), 662–663.
Rheinberger, H.-J. (1997). Toward a history of epistemic things: Synthesizing proteins in the test tube. Stanford: Stanford University Press.
Robertson, R. (1960). Ion transport and respiration. Biological Reviews, 35(2), 231–264.
Robinson, J. D. (1997). Moving questions: A history of membrane transport and bioenergetics. New York: Oxford University Press.
Ruben, S., & Kamen, M. D. (1941). Long-lived radioactive carbon: C14. Physical Review, 59, 349–354.
Sagan, L. (1967). On the origin of mitosing cells. Journal of Theoretical Biology, 14(3), 225–274.
Schaffner, K. F. (1994). Discovery and explanation in biology and medicine. Chicago: University of Chicago Press.
Schickore, J. (2014). Scientific Discovery. In E. N. Zalta (Ed.), The Stanford Encyclopedia of Philosophy (Spring 2014 ed.). http://plato.stanford.edu/archives/spr2014/entries/scientific-discovery/.
Schickore, J., & Steinle, F. (2006). Revisiting discovery and justification: Historical and philosophical perspectives on the context distinction. New York: Springer.
Scholl, R. (2013). Causal inference, mechanisms, and the Semmelweis case. Studies in History and Philosophy of Science, 44(1), 66–76.
Simon, H. A. (1973). Does scientific discovery have a logic? Philosophy of Science, 40(4), 471–480.
Singleton, R. (1997). Heterotrophic \({\text{ CO}}_2\)-fixation, mentors, and students: The Wood-Werkman reactions. Journal of the History of Biology, 30, 91–120.
Skou, J. C. (1957). The influence of some cations on an adenosine triphosphatase from peripheral nerves. Biochimica et biophysica acta, 23, 394–401.
Skou, J. C. (1961). The relationship of a (\({\rm Mg}^{2+} + {\rm Na}^{+}\))-activated, \({\rm K}^{+}\)-stimulated enzyme or enzyme system to the active, linked transport of a \({\rm Na}^{+}\) and \({\rm K}^{+}\) across the cell membrane. In A. Kleinzeller & A. Kotyk (Eds.), Membrane transport and metabolism. New York: Academic Press.
Slater, E. (1953). Mechanism of phosphorylation in the respiratory chain. Nature, 172(4387), 975–978.
Slater, E. (1994). Peter Dennis Mitchell. 29(September), pp. 1920–10, April 1992. Biographical Memoirs of Fellows of the Royal Society, 40, 283–305.
Steinle, F. (2006). Concept formation and the limits of justification: “Discovering” the two electricities. In J. Schickore & F. Steinle (Eds.), Revisiting discovery and justification: Historical and philosophical perspectives on the context distinction (pp. 183–195). New York: Springer.
Thagard, P. (2003). Pathways to biomedical discovery. Philosophy of Science, 70(2), 235–254.
Thimann, K. V. (1938). The absorption of carbon dioxide in photosynthesis. Science, 88, 506–507.
Watson, J., & Crick, F. (1953). Molecular structure of nucleic acids: A structure for deoxyribose nucleic acid. Nature, 171, 737–738.
Weber, B. (1991). Glynn and the conceptual development of the chemiosmotic theory: A retrospective and prospective view. Bioscience Reports, 11(6), 577–617.
Weber, M. (2002). Theory testing in experimental biology: The chemiosmotic mechanism of ATP synthesis. Studies in History and Philosophy of Science Part C: Studies in History and Philosophy of Biological and Biomedical Sciences, 33(1), 29–52.
Weber, M. (2005). Philosophy of experimental biology. Cambridge: Cambridge University Press.
Wilson, A. T., & Calvin, M. (1955). The photosynthetic cycle. \({\text{ CO}_2}\) dependent transients. Journal of the American Chemical Society, 77, 5948–5957.
Wood, H. G., & Werkman, C. H. (1935). The utilization of \({\text{ CO}_2}\) by the propionic acid bacteria in the dissimilation of glycerol. Journal of Bacteriology, 30, 332.
Wood, H. G., & Werkman, C. H. (1936). The utilization of \({\text{ CO}_2}\) in the dissimilation of glycerol by the propionic acid bacteria. Biochemical Journal, 30, 48–53.
Wood, H. G., & Werkman, C. H. (1938). The utilization of \({\text{ CO}_2}\) by the propionic acid bacteria. Biochemical Journal, 32, 1262–1271.
Acknowledgments
We thank the Lake Geneva Biology Interest Group (LG-BIG) and the participants of a January 2014 workshop on “Causality in the Biological Sciences” in Cologne, Germany. In addition, we are particularly grateful to the following for a close reading of an earlier version of the manuscript: Bill Bechtel, Ingo Brigandt, Sara Green and Nick Jones. Raphael Scholl was supported by a Grant from the Swiss National Science Foundation (P300P1_154590).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Scholl, R., Nickelsen, K. Discovery of causal mechanisms. HPLS 37, 180–209 (2015). https://doi.org/10.1007/s40656-015-0061-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40656-015-0061-2