Abstract
Background
Plasmin has recently been reported to be associated with renal fibrosis in experimental models, but its role in human renal diseases is unclear.
Methods
Fifty-seven patients with IgA nephropathy (IgAN) were evaluated retrospectively. Plasmin in their renal biopsy tissues was assessed by in situ zymography using a plasmin-sensitive synthetic peptide, and the relationships between patients’ histologic or clinical parameters and their renal plasmin activity [assessed semiquantitatively by calculating the positively stained percentage of the total tubulointerstitial (TI) area] were evaluated.
Results
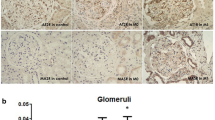
Plasmin activity was observed almost exclusively in the TI space (mainly in the interstitium and partly in the tubular epithelial cells) and was significantly stronger in patients with TI lesion (tubular atrophy/interstitial fibrosis and tubulointerstitial inflammation) than in those without TI lesion. It was significantly and positively correlated with the global glomerulosclerosis rate and significantly and negatively correlated with estimated glomerular filtration rate not only at the time of renal biopsy but also at the end of the follow-up period. Double stainings for plasmin activity and inflammatory cells, cytokeratin, or α-smooth muscle actin (α-SMA) in selected patients revealed TI infiltration of inflammatory cells, attenuated tubular epithelial expression of cytokeratin, and augmented interstitial expression of α-SMA close to upregulated plasmin activity in the TI space.
Conclusions
These data suggest that TI plasmin is associated with TI inflammation leading to renal fibrosis, and can cause the decline in renal function seen in patients with IgAN. Reducing plasmin in situ may therefore be a promising therapeutic approach slowing renal fibrogenesis and improving renal function.






Similar content being viewed by others
References
Vassalli JD, Sappino AP, Belin D (1991) The plasminogen activator/plasmin system. J Clin Invest 88:1067–1072
Oda T, Jung YO, Kim HS et al (2001) PAI-1 deficiency attenuates the fibrogenic response to ureteral obstruction. Kidney Int 60:587–596
Zhang G, Kernan KA, Collins SJ et al (2007) Plasmin(ogen) promotes renal interstitial fibrosis by promoting epithelial-to-mesenchymal transition: role of plasmin-activated signals. J Am Soc Nephrol 18:846–859
Omasu F, Oda T, Yamada M et al (2007) Effects of pioglitazone and candesartan on renal fibrosis and the intrarenal plasmin cascade in spontaneously hypercholesterolemic rats. Am J Physiol Renal Physiol 293:F1292–F1298
Syrovets T, Lunov O, Simmet T (2012) Plasmin as a proinflammatory cell activator. J Leukoc Biol 92:509–519
Li Q, Ke F, Zhang W et al (2011) Plasmin plays an essential role in amplification of psoriasiform skin inflammation in mice. PLoS One 6:e16483
Laumonnier Y, Syrovets T, Burysek L et al (2006) Identification of the annexin A2 heterotetramer as a receptor for the plasmin-induced signaling in human peripheral monocytes. Blood 107:3342–3349
Burysek L, Syrovets T, Simmet T (2002) The serine protease plasmin triggers expression of MCP-1 and CD40 in human primary monocytes via activation of p38 MAPK and janus kinase (JAK)/STAT signaling pathways. J Biol Chem 277:33509–33517
Montrucchio G, Lupia E, De Martino A et al (1996) Plasmin promotes an endothelium-dependent adhesion of neutrophils. Involvement of platelet activating factor and P-selectin. Circulation 93:2152–2160
Lupia E, Del Sorbo L, Bergerone S et al (2003) The membrane attack complex of complement contributes to plasmin-induced synthesis of platelet-activating factor by endothelial cells and neutrophils. Immunology 109:557–563
Eddy AA (2009) Serine proteases, inhibitors and receptors in renal fibrosis. Thromb Haemost 101:656–664
Yoshizawa N, Yamakami K, Fujino M et al (2004) Nephritis-associated plasmin receptor and acute poststreptococcal glomerulonephritis: characterization of the antigen and associated immune response. J Am Soc Nephrol 15:1785–1793
Oda T, Yamakami K, Omasu F et al (2005) Glomerular plasmin-like activity in relation to nephritis-associated plasmin receptor in acute poststreptococcal glomerulonephritis. J Am Soc Nephrol 16:247–254
Levy M, Berger J (1988) Worldwide perspective of IgA nephropathy. Am J Kidney Dis 12:340–347
Manno C, Strippoli GF, D’Altri C et al (2007) A novel simpler histological classification for renal survival in IgA nephropathy: a retrospective study. Am J Kidney Dis 49:763–775
Hotta O, Miyazaki M, Furuta T et al (2001) Tonsillectomy and steroid pulse therapy significantly impact on clinical remission in patients with IgA nephropathy. Am J Kidney Dis 38:736–743
Hotta O, Furuta T, Chiba S et al (2002) Regression of IgA nephropathy: a repeat biopsy study. Am J Kidney Dis 39:493–502
Cattran DC, Coppo R, Cook HT et al (2009) The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int 76:534–545
Roberts IS, Cook HT, Troyanov S et al (2009) The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int 76:546–556
Oda T, Tamura K, Yoshizawa N et al (2008) Elevated urinary plasmin activity resistant to alpha2-antiplasmin in acute poststreptococcal glomerulonephritis. Nephrol Dial Transplant 23:2254–2259
Muller GA, Zeisberg M, Strutz F (2000) The importance of tubulointerstitial damage in progressive renal disease. Nephrol Dial Transplant 15(Suppl 6):76–77
Risdon RA, Sloper JC, De Wardener HE (1968) Relationship between renal function and histological changes found in renal-biopsy specimens from patients with persistent glomerular nephritis. Lancet 2:363–366
Okon K (2003) Tubulo-interstitial changes in glomerulopathy.II. Prognostic significance. Pol J Pathol 54:163–169
Liu Y (2004) Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol 15:1–12
Zeisberg M, Neilson EG (2009) Biomarkers for epithelial-mesenchymal transitions. J Clin Invest 119:1429–1437
Eddy AA (2002) Plasminogen activator inhibitor-1 and the kidney. Am J Physiol Renal Physiol 283:F209–F220
Zhang G, Eddy AA (2008) Urokinase and its receptors in chronic kidney disease. Front Biosci 13:5462–5478
Andersen RF, Buhl KB, Jensen BL et al (2013) Remission of nephrotic syndrome diminishes urinary plasmin content and abolishes activation of ENaC. Pediatr Nephrol 28:1227–1234
Buhl KB, Friis UG, Svenningsen P et al (2012) Urinary plasmin activates collecting duct ENaC current in preeclampsia. Hypertension 60:1346–1351
Plow EF, Herren T, Redlitz A et al (1995) The cell biology of the plasminogen system. FASEB J 9:939–945
Acknowledgments
We thank our colleague Ms. Toshie Fujiwara for expert secretarial assistance.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval
The study protocols were approved by the Ethical Committee of National Defense Medical College.
Informed consent
Informed consent was obtained from each participating patient in accordance with the principles of the Declaration of Helsinki.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Uchida, T., Oda, T., Takechi, H. et al. Role of tubulointerstitial plasmin in the progression of IgA nephropathy. J Nephrol 29, 53–62 (2016). https://doi.org/10.1007/s40620-015-0205-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-015-0205-1