Abstract
Objective
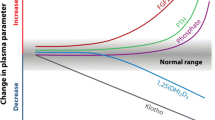
It is uncertain whether increasing 25-hydroxyvitamin D (25-D) levels in chronic kidney disease (CKD) patients above those recommended by current guidelines result in progressive amelioration of secondary hyperparathyroidism. Our objective was to identify a potential therapeutic 25-D target which optimally lowers plasma parathyroid hormone (PTH) without producing excessive hypercalcemia or hyperphosphatemia in CKD.
Methods
We performed a cross-sectional analysis of 14,289 unselected stage 1–5 CKD patients from US primary care and nephrology practices utilizing a laboratory-based CKD clinical decision support service between September 2008 and May 2012. Estimated glomerular filtration rate (eGFR), plasma PTH, and serum 25-D, calcium, and phosphorus results were analyzed.
Results
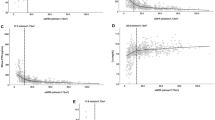
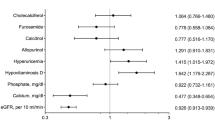
In CKD stages 3–5, progressively higher 25-D pentiles contained progressively lower mean PTH levels. Regression analysis of log PTH on 25-D was significant in all CKD stages with no evidence of a decreasing effect of 25-D to lower PTH until 25-D levels of 42–48 ng/ml. Progressively higher 25-D concentrations were not associated with increased rates of hypercalcemia or hyperphosphatemia.
Conclusions
We found evidence for an optimal level of 25-D above which suppression of PTH progressively diminishes. This level is more than twice that currently recommended for the general population. We found no association between these higher 25-D levels and hyperphosphatemia or hypercalcemia. Additional prospective trials seem appropriate to test the idea that 25-D levels around 40–50 ng/ml could be a safe and effective treatment target for secondary hyperparathyroidism in CKD.




Similar content being viewed by others
References
Ennis J, Worcester E, Coe F (2012) Contribution of calcium, phosphorus and 25-hydroxyvitamin D to the excessive severity of secondary hyperparathyroidism in African-Americans with CKD. Nephrol Dial Transplant 27:2847–2853
Levin A, Bakris GL, Molitch M et al (2007) Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int 71:31–38
De Boer IH, Gorodetskaya I, Young B, Hsu CY, Chertow GM (2002) The severity of secondary hyperparathyroidism in chronic renal insufficiency is GFR-dependent, race-dependent, and associated with cardiovascular disease. J Am Soc Nephrol 13:2762–2769
Vassalotti JA, Uribarri J, Chen SC et al (2008) Trends in mineral metabolism: Kidney Early Evaluation Program (KEEP) and the National Health and Nutrition Examination Survey (NHANES) 1999–2004. Am J Kidney Dis 51:S56–S68
Gutierrez OM, Isakova T, Andress DL, Levin A, Wolf M (2008) Prevalence and severity of disordered mineral metabolism in Blacks with chronic kidney disease. Kidney Int 73:956–962
Reichel H, Deibert B, Schmidt-Gayk H, Ritz E (1991) Calcium metabolism in early chronic renal failure: implications for the pathogenesis of hyperparathyroidism. Nephrol Dial Transplant 6:162–169
Ishimura E, Nishizawa Y, Inaba M et al (1999) Serum levels of 1,25-dihydroxyvitamin D, 24,25-dihydroxyvitamin D, and 25-hydroxyvitamin D in nondialyzed patients with chronic renal failure. Kidney Int 55:1019–1027
Pitts TO, Piraino BH, Mitro R et al (1988) Hyperparathyroidism and 1,25-dihydroxyvitamin D deficiency in mild, moderate, and severe renal failure. J Clin Endocrinol Metab 67:876–881
Zisman AL, Hristova M, Ho LT, Sprague SM (2007) Impact of ergocalciferol treatment of vitamin D deficiency on serum parathyroid hormone concentrations in chronic kidney disease. Am J Nephrol 27:36–43
Colodro IH, Brickman AS, Coburn JW, Osborn TW, Norman AW (1978) Effect of 25-hydroxy-vitamin D3 on intestinal absorption of calcium in normal man and patients with renal failure. Metabolism 27:745–753
Eastwood JB, Stamp TC, Harris E, de Wardener HE (1976) Vitamin-D deficiency in the osteomalacia of chronic renal failure. Lancet 2:1209–1211
Kandula P, Dobre M, Schold JD, Schreiber MJ Jr, Mehrotra R, Navaneethan SD (2011) Vitamin D supplementation in chronic kidney disease: a systematic review and meta-analysis of observational studies and randomized controlled trials. Clin J Am Soc Nephrol 6:50–62
Kidney Disease: Improving Global Outcomes (KDIGO) CKD–MBD Work Group (2009) KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int 76(Suppl 113):S1–S130
Institute of Medicine (IOM) (2011) Dietary reference intakes for calcium and vitamin D. The National Academies Press, Washington, DC
DeVille J, Thorp ML, Tobin L, Gray E, Johnson ES, Smith DH (2006) Effect of ergocalciferol supplementation on serum parathyroid hormone and serum 25-hydroxyvitamin D in chronic kidney disease. Nephrology (Carlton) 11:555–559
Al-Aly Z, Qazi RA, Gonzalez EA, Zeringue A, Martin KJ (2007) Changes in serum 25-hydroxyvitamin D and plasma intact PTH levels following treatment with ergocalciferol in patients with CKD. Am J Kidney Dis 50:59–68
Oksa A, Spustova V, Krivosikova Z et al (2008) Effects of long-term cholecalciferol supplementation on mineral metabolism and calciotropic hormones in chronic kidney disease. Kidney Blood Press Res 31:322–329
Dogan E, Erkoc R, Sayarlioglu H, Soyoral Y, Dulger H (2008) Effect of depot oral cholecalciferol treatment on secondary hyperparathyroidism in stage 3 and stage 4 chronic kidney diseases patients. Ren Fail 30:407–410
Chandra P, Binongo JN, Ziegler TR et al (2008) Cholecalciferol (vitamin D3) therapy and vitamin D insufficiency in patients with chronic kidney disease: a randomized controlled pilot study. Endocr Pract 14:10–17
Moe SM, Saifullah A, LaClair RE, Usman SA, Yu Z (2010) A randomized trial of cholecalciferol versus doxercalciferol for lowering parathyroid hormone in chronic kidney disease. Clin J Am Soc Nephrol 5:299–306
Trakarnvanich T, Chalapipat O, Disthabanchong S et al (2010) Effect of high dose ergocalciferol in chronic kidney disease patients with 25-hydroxyvitamin D deficiency. J Med Assoc Thai 93:885–891
Qunibi WY, Abdellatif A, Sankar S et al (2010) Treatment of vitamin D deficiency in CKD patients with ergocalciferol: are current K/DOQI treatment guidelines adequate? Clin Nephrol 73:276–285
Kovesdy CP, Lu JL, Malakauskas SM, Andress DL, Kalantar-Zadeh K, Ahmadzadeh S (2012) Paricalcitol versus ergocalciferol for secondary hyperparathyroidism in CKD stages 3 and 4: a randomized controlled trial. Am J Kidney Dis 59:58–66
Alvarez JA, Law J, Coakley KE et al (2012) High-dose cholecalciferol reduces parathyroid hormone in patients with early chronic kidney disease: a pilot, randomized, double-blind, placebo-controlled trial. Am J Clin Nutr 96:672–679
Marckmann P, Agerskov H, Thineshkumar S et al (2012) Randomized controlled trial of cholecalciferol supplementation in chronic kidney disease patients with hypovitaminosis D. Nephrol Dial Transplant 27:3523–3531
Holick MF (2007) Vitamin D deficiency. N Engl J Med 357:266–281
LaClair RE, Hellman RN, Karp SL et al (2005) Prevalence of calcidiol deficiency in CKD: a cross-sectional study across latitudes in the United States. Am J Kidney Dis 45:1026–1033
Nigwekar SU, Bhan I, Thadhani R (2012) Ergocalciferol and cholecalciferol in CKD. Am J Kidney Dis 60:139–156
Saab G, Young DO, Gincherman Y, Giles K, Norwood K, Coyne DW (2007) Prevalence of vitamin D deficiency and the safety and effectiveness of monthly ergocalciferol in hemodialysis patients. Nephron Clin Pract 105:c132–c138
Tokmak F, Quack I, Schieren G et al (2008) High-dose cholecalciferol to correct vitamin D deficiency in haemodialysis patients. Nephrol Dial Transplant 23:4016–4020
Conflict of interest
I (J. Ennis) am an employee of Litholink Corporation, a subsidiary of Laboratory Corporation of America (LabCorp), and hold stock in LabCorp. The research conducted was sponsored by this company. E. Worcester, F. Coe, and S. Sprague are consultants for Litholink Corporation and members of the Litholink CKD Scientific Advisory Board. S. Sprague is a consultant for and received honoraria from Amgen, Cytochroma, Kai, and Roche. S. Sprague received grant support from Amgen, Abbott, Cytochroma, NIH, Reata, Shire, and Vifor. All work for this project was undertaken at Litholink Corporation. All authors have made important contributions to the design and execution of this study, the analysis and interpretation of data, and the writing of this manuscript.
Ethical standard
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
For this type of study formal consent is not required.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
40620_2015_186_MOESM1_ESM.jpg
Supplemental Figure S1: Plot of the difference function between linear and cubic regressions of Log Plasma PTH vs 25-D. Coefficients are in Supplemental Table S1. The form of the function is in the footnote to Table S1. Y axis: C = cubic, L = linear predicted values. Zero crossing points are indicated along the horizontal dashed line. The cubic equation departs upward from the linear regression at x values between 42 -48 ng/ml. Stages are represented by panels as in Figure 2 (JPEG 241 kb)
40620_2015_186_MOESM2_ESM.jpg
Supplemental Figure S2: Individual log linear and cubic regressions of Plasma PTH vs 25-D. The crossing points of the linear and cubic equations occur at the points where the difference equation (Figure S1) cross zero. This graph is meant simply to illustrate the 2 regressions visually (JPEG 237 kb)
Rights and permissions
About this article
Cite this article
Ennis, J.L., Worcester, E.M., Coe, F.L. et al. Current recommended 25-hydroxyvitamin D targets for chronic kidney disease management may be too low. J Nephrol 29, 63–70 (2016). https://doi.org/10.1007/s40620-015-0186-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-015-0186-0