Abstract
Background
Colistin pharmacokinetics data are scarce regarding patients undergoing renal replacement therapy (RRT), or even absent as in patients treated with sorbent technologies potentially capable of removing colistin by extensive absorption on many polymeric materials.
Methods
Twelve septic shock patients with acute kidney injury (AKI) undergoing RRT [continuous venovenous hemodiafiltration (CVVHDF) n = 7, coupled-plasma filtration adsorption-HF (CPFA-HF) n = 4, hemoperfusion n = 1] treated with colistin methanesulfonate at a dose of 4.5 × 106 U bid were studied. Colistin A (Col-A) and colistin B (Col-B) concentrations on plasma and effluent at time 0, 0.2, 1, 3, 6, 12, 24 and 48 h were determined by the liquid chromatography-tandem mass spectrometry method.
Results
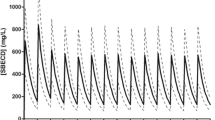
With CVVHDF the sieving coefficient was lower for Col-A, peaked early (0.40 for Col-A at 10 min, and 0.59 for Col-B at 3 h) and declined after 48 h (0.22 and 0.30 for Col-A and Col-B, respectively). Colistin’s filter clearance showed a similar pattern, with the highest clearance value of 18.7 ml/min for Col-B at 1 h. With CPFA-HF after the cartridge the Col-A and Col-B levels were negligible (<0.2 mg/l) or not detectable. The sum of the effluent and cartridge clearances reached values of 30 and 40 ml/min for Col-A and Col-B, respectively. With hemoperfusion the postcartridge concentrations for Col-A and Col-B were about 30 % lower than those determined precartridge.
Conclusions
During CPFA-HF and CVVHDF, the extent of colistin removal is high, and patients should receive an unreduced dosage. However, due to risk of accumulation in long-term administration colistin plasma levels determination is recommended.





Similar content being viewed by others
References
Wareham DW, Bean DC, Khanna P et al (2008) Bloodstream infection due to Acinetobacter spp.: epidemiology, risk factors and impact of multi-drug resistance. Eur J Clin Microbiol Infect Dis 27:607–612
Li J, Nation RL, Turnidge JD, Milne RW, Coulthard K, Rayner CR, Paterson DL (2006) Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect Dis 6:589–601
Falagas ME, Kasiakou SK (2005) Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin Infect Dis 40:1333–1341
Bergen PJ, Landersdorfer CB, Zhang J et al (2012) Pharmacokinetics and pharmacodynamics of ‘old’ polymyxins: what is new? Diagn Microbiol Infect Dis 74:213–223
Koch-Weser J, Sidel VW, Federman EB, Kanarek P, Finer DC, Eaton AE (1970) Adverse effects of sodium colistimethate. Manifestations and specific reaction rates during 317 courses of therapy. Ann Intern Med 72:857–868
Hartzell JD, Neff R, Ake J et al (2009) Nephrotoxicity associated with intravenous colistin (colistimethate sodium) treatment at a tertiary care medical center. Clin Infect Dis 48:1724–1728
Collins JM, Haynes K, Gallagher JC (2013) Emergent renal dysfunction with colistin pharmacotherapy. Pharmacotherapy 33:812–816
Sorlí L, Luque S, Grau S et al (2013) Trough colistin plasma level is an independent risk factor for nephrotoxicity: a prospective observational cohort study. BMC Infect Dis 13:380
Li J, Rayner CR, Nation RL, Deans R et al (2005) Pharmacokinetics of colistin methanesulfonate and colistin in a critically ill patient receiving continuous venovenous hemodiafiltration. Antimicrob Agents Chemother 49:4814–4815
Marchand S, Frat JP, Petitpas F et al (2010) Removal of colistin during intermittent haemodialysis in two critically ill patients. J Antimicrob Chemother 65:1836–1837
Garonzik SM, Li J, Thamlikitkul V, Paterson DL et al (2011) Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother 55:3284–3294
Healy DP, Sombun AD, Gardner JC et al (2011) Pharmacokinetics of colistin in an adolescent boy with extensive burn injury. J Burn Care Res 32:e7–e11
Markou N, Fousteri M, Markantonis SL et al (2012) Colistin pharmacokinetics in intensive care unit patients on continuous venovenous haemodiafiltration: an observational study. J Antimicrob Chemother 67:2459–2462
Karvanen M, Plachouras D, Friberg LE et al (2013) Colistin methanesulfonate and colistin pharmacokinetics in critically ill patients receiving continuous venovenous hemodiafiltration. Antimicrob Agents Chemother 57:668–671
Honore PM, Jacobs R, Lochy S et al (2013) Acute respiratory muscle weakness and apnea in a critically ill patient induced by colistin neurotoxicity: key potential role of hemoadsorption elimination during continuous venovenous hemofiltration. Int J Nephrol Renovasc Dis 6:107–111
Luque S, Sorli L, Li J et al (2014) Effective removal of colistin methanesulphonate and formed colistin during intermittent hemodialysis in a patient infected by polymyxin-only-susceptible Pseudomonas aeruginosa. J Chemother 26:122–124
Ronco C, Inguaggiato P, D’Intini V et al (2003) The role of extracorporeal therapies in sepsis. J Nephrol 16(Suppl 7):S34–S41
Livigni S, Bertolini G, Rossi C, On behalf of GiViTI et al (2014) Efficacy of coupled plasma filtration adsorption (CPFA) in patients with septic shock: a multicenter randomised controlled clinical trial. BMJ Open 4(1):e003536
Cruz DN, Perazella MA, Bellomo R et al (2007) Effectiveness of polymyxin B-immobilized fiber column in sepsis: a systematic review. Crit Care 11(2):R47
Matzke GR, Aronoff GR, Atkinson AJ Jr et al (2011) Drug dosing consideration in patients with acute and chronic kidney disease-a clinical update from kidney disease: improving global outcomes (KDIGO). Kidney Int 80:1122–1137
Leporati M, Bua RO, Mariano F et al (2014) Determination by LC-MS/MS of colistins A and B in plasma and ultrafiltrate from critically ill patients undergoing continuous venovenous hemodiafiltration. Ther Drug Monit 36:182–189
Dellinger RP, Levy MM, Rhodes A et al (2013) Surviving sepsis campaign guidelines committee including the pediatric subgroup. surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 39:165–228
Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, Acute Dialysis Quality Initiative workgroup (2004) Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) group. Crit Care 8:R204–R212
Mariano F, Tedeschi L, Morselli M, Stella M, Triolo G (2010) Normal citratemia and metabolic tolerance of citrate anticoagulation for hemodiafiltration in severe septic shock burn patients. Intensive Care Med 36:1735–1743
Mariano F, Tetta C, Stella M, Miletto A, Triolo G (2004) Regional citrate anticoagulation in critically ill patients treated with plasma filtration and adsorption. Blood Purif 22:313–319
Plachouras D, Karvanen M, Friberg LE et al (2009) Population pharmacokinetic analysis of colistin methanesulfonate and colistin after intravenous administration in critically ill patients with infections caused by gram-negative bacteria. Antimicrob Agents Chemother 53:3430–3436
Guideline on bioanalytical method validation (2011). European Medicines Agency. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/08/WC500109686.pdf (553 EMEA/CHMP/EWP/192217/2009)
Schmidt JJ, Hafer C, Clajus C et al (2012) New high-cutoff dialyzer allows improved middle molecule clearance without an increase in albumin loss: a clinical crossover comparison in extended dialysis. Blood Purif 34:246–252
Heilmann K, Keller T (2011) Polysulfone: the development of a membrane for convective therapies. Contrib Nephrol 175:15–26
Couet W, Grégoire N, Gobin P et al (2011) Pharmacokinetics of colistin and colistimethate sodium after a single 80-mg intravenous dose of CMS in young healthy volunteers. Clin Pharmacol Ther 89:875–879
Strunk AK, Schmidt JJ, Baroke E, Bode-Böger SM, Martens-Lobenhoffer J, Welte T, Kielstein JT (2014) Single- and multiple-dose pharmacokinetics and total removal of colistin in a patient with acute kidney injury undergoing extended daily dialysis. J Antimicrob Chemother 69:2008 (Epub ahead of print) (PubMed PMID: 24651826)
Harm S, Falkenhagen D, Hartmann J (2014) Endotoxin adsorbents in extracorporeal blood purification: do they fulfill expectations? Int J Artif Organs 37:222–232
Acknowledgments
For this study we did not receive any specific financial support.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mariano, F., Leporati, M., Carignano, P. et al. Efficient removal of colistin A and B in critically ill patients undergoing CVVHDF and sorbent technologies. J Nephrol 28, 623–631 (2015). https://doi.org/10.1007/s40620-014-0143-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-014-0143-3