Abstract
Green biotechnology related to biomass conversion for production of bio-based chemicals has drawn considerable attention because of the increasing costs of fossil fuels and their adverse effects on climate change, environmental pollution, and human health. In this study, the recent promises and issues of low-carbon-driven bio-product technology development using lignocellulosic substrates from agriculture were critically reviewed. First, the challenges in bio-based chemical production were addressed from the aspect of technology. After that, the lignocellulose feedstock and their building-block chemicals used in the literature were summarized. In addition, novel pretreatment, bio-conversion, and separations technologies for cleaner production of bio-based products were comprehensively reviewed. It suggests that the main challenge to deploying bio-based chemical technologies into commercialization is the high cost of product recovery from the bioreactor due to the relatively low product titers caused by the product inhibition and/or pH in conventional fermentation.
Similar content being viewed by others
Introduction
Fossil-based chemicals are produced using compounds from crude oil, coal, or natural gas with the aid of co-reagents, such as ammonia, and multiple process steps. In contrast, biomass containing a great amount of proteins, amino acids, and lignocellulose (such as agricultural wastes, forestry residues, grasses, and woody materials) is a renewable and inexpensive feedstock for the production of fuels, chemicals, materials, and heat/power. Adom et al. [1] found that the bio-products can uniformly offer great reductions in greenhouse gas (GHG) emissions ranging from 39 to 86 %, compared to their fossil counterparts, based on a cradle-to-grave life cycle assessment. Tsiropoulos et al. [2] also reported that bio-based chemicals, for example polyethylene, result in decreasing greenhouse gas emissions of ∼0.75 kg CO2-eq per kg of polyethylene production, i.e., 140 % lower than petrochemical polyethylene, and saving non-renewable energy use for ∼65 %. Therefore, bio-based chemicals production in biorefineries using biomass feedstocks is an attractive alternative due to its environmental benefits such as carbon neutral property.
Bio-based technologies and products, industrial biotechnology, are a key opportunity for significant green growth. The OECD [3] reported that biotechnology could contribute to 2.7 % of GDP in 2030 within the OECD region, and make the largest economic contribution in industry and primary production. However, there are challenges facing the continued expansion of industrial biotechnology such as sustainable availability of raw materials with increasing climate and severe weather impacts, water availability, and stability of the markets [4].
Biomass can be converted into high-value bio-based chemicals and products including biofuels, organic acids, amino acids, sugar alcohols, fatty acids, bio-polymers, bio-monomers, bio-pharmaceuticals, and cheap energy sources for microbial fermentation and enzyme production [4–6]. Conceptually, the bio-refinery industry applies a hybrid collection of core technologies from different fields including chemistry, bio-engineering, and agricultural expertise, which generally encompasses two major platforms: thermochemical conversion and biochemical conversion. Several types of bio-technologies such as using a microbial electrochemical method [7] have been operated at a large scale or in industrial manufacturing. Other emerging technologies for processing biomass include microwave-assisted biorefining [8], supercritical fluid extraction [9, 10], oxidation methods [11], and resin-wafer electrodeionization with a separative bioreactor (RW-EDI/SB) [12].
In this study, we address the challenges in bio-based chemicals production from the aspect of technology. The available biomass feedstocks and their building-block chemicals used in the literature were also summarized. In addition, we review breakthroughs in novel pretreatment, bio-conversion, and separations technologies for cleaner production of bio-based products.
Challenges in Bio-refinery Technologies
Normally, the conversion of lignocellulose biomass into building-block chemicals for further value-added product synthesis requires a multi-step procedure including (1) pretreatment and conditioning, (2) conversion process, and (3) separations and purification. The pretreatment processes include dilute acid method, ammonium additive, two-stage process, and microwave. The common conversion processes include gasification, pyrolysis, hydrothermal liquefaction, catalysis, enzymatic hydrolysis, and fermentation. Recovery and purification are complicated steps in fermentation processes and can be the most costly process in the bio-product manufacturing. They usually depend on both the nature of the product and the complexity of the fermentation broth [13]. For example, recovery of xylitol from lignocellulosic media fermented by microorganisms as well as the possible viable techniques for downstream processes is poorly explored in scientific papers [13].
There are several key technology challenges in all bio-refinery processes. Logistics costs are a major hurdle across the bio-refinery industry. One approach is to use a distributed pretreatment scheme to process close to the origin of the biomass resource, thereby increasing the biomass density. Second, efficient conversion of lignocellulose biomass into products is another major hurdle due to the complexity of lignocellulosic biomass structure. As a result, the development of new reactor technology for continuous operation is critical for efficient biomass processing [14]. Third, the dilute (watery) waste streams need to be managed throughout the process. A key strategy is to develop a simultaneous (in situ) conversion/separations process for main components or derivatives at low cost, and low resource (especially water) and energy consumption. To improve transformation yields and rates, new platform chemicals should be identified that can be derived at sufficient volumes, yields, and energy efficiency to control costs.
There is strong public and private momentum to improve the sustainability of bio-chemicals production. Strategies for economic and environmental production include (1) efficient bioprocesses, (2) bio-catalysis without product inhibition, and (3) product recovery. According to Anwar et al. [15], advanced bio-technologies should be focused on discovery and characterization of new enzymes, and production in homologous or heterologous systems.
Lignocellulose Feedstock from Agriculture and Forestry
Biomass as used in this article refers to the organic matter originated from organisms such as energy crops (e.g., corn and rice straw), agricultural and forestry wastes (e.g., pod and bagasse), domestic waste (e.g., kitchen waste and brown grease), animal husbandry wastes (e.g., carcass), and industrial wastes (e.g., rubbers and paper). Lignocellulosic feedstocks, or second-generation feedstocks, are the most abundant renewable organic resource available and are composed of sugars and lignin [16•]. Agricultural lignocellulosic biomass comes from the wastes or residues in agricultural or forestry processing, such as sugarcane bagasse [17, 18], corn stover [19], beechwood [20], sweet sorghum [21], corncob [22], rice straw [11, 23], nutshells and pomace [24], palm empty fruit bunches [25], wheat straw [26], and onion/potato waste [27].
Table 1 presents the composition of selected lignocellulosic biomass types associated with their treatment process in the literature. Two main types of agricultural biomass feedstock (i.e., sugar-rich and lignin-rich) can be converted under benign conditions, normally not at high temperatures and pressures, to produce desired products with limited byproducts. Polysaccharides sourced from starch (C6H10O5) n derived from lignocellulosic biomass are most easily processed to glucose via hydrolysis [14]. Therefore, research on the starch-based biomass has been extensively studied to achieve a cost-effective process for bio-based chemicals or fuels. Lignocellulose is a biopolymer comprising of 30–50 % cellulose, 20–40 % hemicellulose, and 10–25 % lignin [14, 15], along with smaller quantities of other organic and non-organic compounds such as proteins, lipids, and other extractives [29•].
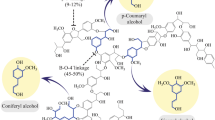
Cellulose (C6H10O5) n , the main constituent of plant cell walls, is one of the most abundant polymers on the planet. It is a complex polysaccharide, consisting of 3000 or more β-(1/4) linked d-glucose units [16•]. Hemicellulose (C5H8O5) n , in contrast, is a relatively amorphous and highly branched heteropolysaccharide, consisting of a wide variety of C5 and C6 sugars, which is easier to chemically or thermally decompose than cellulose. On the other hand, lignin, a phenylpropane-based polymer, contains three aromatic alcohols (coniferyl alcohol, sinapyl alcohol, and p-coumaryl alcohol) produced through a biosynthetic process and forms a protective seal around the cellulose and hemicelluloses. Cross-linking of the cellulose and hemicellulosic components with lignin via ester and ether linkages renders lignocellulose resistant towards hydrolysis [14].
Lammens et al. [30] have assessed the biomass source availability of proteins (e.g., amino acid) for the production of bulk chemicals in a bio-refinery. Their results indicate that glutamic acid is the most abundant protein building block in almost all investigated byproduct streams, except for sugarcane vinasse, as starting materials for chemical products. It can be a source for the production of bio-based pyrrolidone derivatives such as N-methylpyrrolidone (NMP) and N-vinylpyrrolidone (NVP), the polyamide precursor succinonitrile, and acrylonitrile. According to their estimate, there are enough sources available to produce bio-based chemicals such as NMP with market sizes around 100 k tonnes per year from amino acids. Bulk chemicals (e.g., acrylonitrile) can be completely replaced by their bio-based equivalent, by expanding the production of biomass such as grasses [30].
Important Building Blocks for Bio-chemicals Production
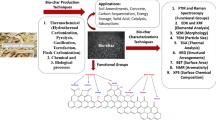
Figure 1 shows the schematic diagram of lignocellulose substrate utilization for bio-based chemical production, which indicates that possible building blocks (platform chemicals) from biomass include succinic acid, fumaric acid, maleic acid, levulinic acid, glucaric acid, itaconic acid, aspartic acid, glutamic acid, glycerol, sorbitol, and xylitol [12, 14]. One class of chemical products that are produced in large volumes via bio-catalysis process are organic acids such as gluconic and lactic acids. For example, acidogenic microorganisms anaerobically convert lignocellulosic biomass into short- and medium-chain fatty (also called carboxylic or organic) acids, such as lactic, formic, acetic, propionic, butyric, valeric, and hexanoic acids [31]. Those products themselves are valuable building-block chemicals, which can be further synthesized into value-added products via various routes.
The investment and growth in production capacity of bio-based materials continue to increase globally. According to an estimation from Lux Research, building-block chemicals such as adipic acid and lactic acid are growing from 2.0 M tonnes in 2013 to 4.9 M tonnes in 2017 [32]. In 2013, of the commercial bio-based chemicals production (excluding ethanol), short-chain fatty acids accounted for 46 % in total global bio-based chemical production capacity, followed by sorbitol at approximately 16 %, glycerin at 14 %, and fatty alcohols production at 11 % [33]. Lactic acid, furfural, and several other small-volume chemicals rounded out the bio-based production capacity for 2013.
Bio-based organic acids production typically results in only dilute product concentrations because product inhibition and acidification drive pH outside of the optimal range for the bio-catalyst [34]. Therefore, numerous studies on organic acid purification (separations) have been conducted using techniques such as ion exchange, reactive extraction, membrane separations, distillation, and electrodialysis processes [12, 35]. On the other hand, the use of lignin for chemical production has been limited due to contamination from salts, carbohydrates, particulates, volatiles, and the molecular weight distribution of lignosulfonates [16•].
Novel Pretreatment Processes
Table 2 summarizes the novel pretreatment, bio-conversion, and separations processes in the literature. Pretreatment of lignocellulose substrates is a critical stage for bio-refinery to improve the transformation yields and rates. Since the pretreatment process can break down the lignin structure, disrupt the crystalline structure of cellulose, and increase the porosity (accessible surface area) of the biomass, the acids or enzymes can easily access the cellulose to hydrolyze into monomers. To date, various pretreatment methods, including (1) mechanical, e.g., milling [26]; (2) thermal, e.g., SO2-catalyzed steam explosion [40]; (3) chemical, e.g., dilute acid [41•], two-stage treatment [42], green liquor [43]; and (4) biological, e.g., fermentation [26], have been extensively investigated by different researchers.
The ideal pretreatment process should produce a disrupted and hydrated substrate that is easily hydrolyzed and optimized to accommodate the requirements of subsequent conversion steps, such as (1) minimization of inorganic materials and (2) separation of main constituents lignin, cellulose, and hemicellulose [16•]. Moreover, in a sugar fermentation process, the formation of sugar degradation products and fermentation inhibitors should be avoided. In this study, several novel pretreatment processes including microwave, supercritical fluid extraction, and chemical/oxidation methods are reviewed.
Microwave Technique (Physical)
Although microwave techniques have been operated on a commercial scale since the early 1960s, the specific effects of microwaves on biomass (in particular, on cellulose) have been recognized only recently [44]. The wavelengths of microwave range between 1 and 1000 mm, corresponding to frequency of 300–0.3 GHz, which fall in between infrared and radio wave regions of the electromagnetic spectrum. Since the wavelength range could interfere with radar and communications, the Federal Communications Commission regulates the specific wavelength for industrial, scientific, and medical purposes that, for instance, the available frequencies for industrial equipment are 0.915, 2.45, 5.8, and 24,124 GHz [45]. The penetration depth of microwave into materials is an important parameter in the microwave process design and scaleup, where the penetration depth varies with the types of material, microstructure properties, microwave frequency, and operating temperature [46].
It has been proven that the initial cellulosic polysaccharide helices can be rapidly activated by microwaves, leading to the formation of reactive oligosaccharides for quickly undergoing further chemistry [47]. Su et al. [17] also found that an additional sugarcane breakdown of 5 % was achieved using microwaves. Under microwave irradiation, the production of chemicals from biomass proceeds at markedly lower temperatures (up to 150 °C) compared to conventional heating [8]. Moreover, the molecules with a high degree of functionality can be produced while conventional heating tends to produce a great proportion of lower-value gases. Furthermore, with microwave-assisted heating, the CMF with a purity of 74–98 % can be synthesized using various types of solvents, compared with conventional heating at typically 5–10 % yields of CMF [36].
One of the most significant advantages of microwave pyrolysis is the in situ separation of bio-oil into a minimum of two fractions based on differences in their boiling points. However, there are several challenges in microwave application to biomass processing including (1) control of the decomposition process so that a limited number of reaction pathways occur leading to selective processes for converting biomass into molecular products, (2) development of rapid separation of particular products or intermediates into continuous processing, and (3) optimization of the energy efficiency, economic costs, and environmental benefits [47].
Supercritical Fluid Extraction (Chemical)
From the green chemistry point of view, the attractive process solvents include water (limited to water-soluble compounds), ethanol, and carbon dioxide (CO2) because they are generally non-toxic, non-hazardous, and environmentally degradable. Supercritical CO2 has a critical temperature of 31 °C and a critical pressure of 7.4 MPa. Since CO2 is able to readily convert into the supercritical state and offer a wider range of solvent strengths through the judicial application of different combinations of temperature and pressure, it has been utilized as a supercritical fluid for extraction in applications such as the food industry [47, 48]. Supercritical fluid CO2 extraction (SCF-CO2) has been used to isolate essential components from microalgae such as fatty acids, lipids (e.g., triglycerides), bio-pharmaceuticals, and pigment (e.g., carotenoids) [9, 10]. Because CO2 can be recycled, SCF-CO2 has been evaluated for many processes.
Due to the non-polarity of CO2, it has been considered a suitable solvent for the extraction of lipids, especially from microalgae, and lignin operating below its supercritical pressure [10, 49]. It is predicted that CO2 solubilized in water (carbonic acid) catalyzes the hydrolysis of hemicellulose in biomass [49]. One limitation is that SCF-CO2 reduces the extraction efficiency of polar co-solvents. Water content in microalgae samples is usually high. Extraction may require reduction to below 20 wt% water to achieve the best performance for SCF-CO2 extraction [50].
According to the results by Tibbetts et al. [9], the SCF-CO2 extraction can significantly reduce crude lipid (285.5 g/kg in original biomass) and caloric contents (23.4 MJ/kg in original biomass) of residual biomass to 256.2–256.3 g/kg and 23.0–23.1 MJ/kg, respectively. It was also noted that SCF-CO2 extraction in the presence of water can efficiently improve the enzymatic digestibility of aspen (hardwood) and southern yellow pine (softwood) [49].
Chemical and/or Oxidation Methods
Green liquor, a mixture of sodium carbonate and sodium sulfide, has been recently considered as an ideal pretreatment step because it keeps both cellulose and hemicellulose fractions in the substrate for enzymatic hydrolysis [43]. The hydrolysis yields of glucan and xylan were found to be 84.6 and 77.2 %, respectively, with a solid-to-liquid ratio of 1:6 (w/v) [43].
Jung et al. [11] developed a novel lignocellulose pretreatment using Fenton-mimicking process (FeCl3/H2O2) at relatively high solids loading of biomass. The initiative radicals and iron species from the Fenton reaction will first attack lignin and hemicellulose due to their location in the outer portions of lignocellulose, and produce demethylated, oxidized, or fragmented lignin and polysaccharides. By optimizing reaction conditions, the highest enzymatic digestibility of 93.2 % was obtained from rice straw pretreated with 10 % (w/v) solids loading at 25 °C.
Development of Innovative Bio-conversion and Separations Technologies for Cleaner Production
Fermentation: Genetically Modified Biocatalytic Platform
Metabolic engineering is one of the key thrusts in bio-based production due the capability to synthesize targeted products with high selectivity and atom efficiency. Chen et al. [6] suggests focus on (i) using simple, available, and inexpensive starting materials, (ii) identifying and eliminating bottlenecks in pathways for the desired products, (iii) constructing robust biocatalysts, and (iv) optimizing regulatory networks to maximize yields, titers, and productivity. Fermentation has been extensively used as a pretreatment process and/or bio-conversion process [25]. For instance, the dark fermentation corresponds to the first step of anaerobic digestion (considered as a substrate pretreatment) after inhibition of the methanogenic activity, which simultaneously produces high amount of hydrogen (H2) and valuable volatile fatty acids (VFA). Motte et al. [26] combined dry dark fermentation and mechanical pretreatment of wheat straw prior to bioethanol fermentation, which successfully improve the substrate conversion into bio-products (i.e., biofuels and VFA) of a factor two.
Zhang et al. [28] conducted a pioneering study on a Candida athensensis strain SB18 for xylitol production using batch fermentation and fed-batch fermentation. The results successfully demonstrated that stain C. athensensis SB18 is a promising strain for high-titer and high-yield xylitol production, corresponding to yield and productivity of 0.87 g/g substrate and 256.5 g/L, respectively. Park et al. [39] reported for the pioneering biosynthesis of polyhydroxyalkanoates (PHA) containing different 2-hydroxybutyrate (2HB) monomer fractions, ranging from 10 to 60 mol%, from glucose by metabolically engineered Escherichia coli strains. The highest 2HB fraction in the copolymer was obtained by adding 2 g/L of 2HB and 0.5 g/L of 3HB to the culture medium, which resulted in the production of P (61 mol%2HB-co-36 mol%3HB-co-3 mol%LA) with the PHA content of 26.7 wt%.
On the other hand, over the last 50 years, extensive development of genetic systems for fundamental understanding of the metabolism of E. coli is the prime prokaryotic genetic model [6]. Bio-based chemicals can be produced with engineered E. coli at low production cost because of its rapid doubling time and growth rate, ease of high-cell-density fermentation, and the availability of excellent genetic tools for strain improvement. Zhou et al. [38] have developed an engineered E. coli strain (B0013-070B) to exhibit high overall volumetric productivity and the oxygen-limited productivity of 4.32 and 6.73 g/L/h, respectively. Moreover, the d-lactate productivity was 122.8 g/L, with an increased oxygen-limited productivity of 0.89 g/g/h, revealing the effectiveness of using a genetic switch to regulate cell growth and the production of a metabolic compound. The scaled-up conditions optimized in a shake flask experiment were utilized in the bioreactor experiment.
Anaerobic Co-digestion
Anaerobic digestion (AD) is a naturally occurring, biological pretreatment of organic substrates carried out by microbial communities in the absence of oxygen. However, the conventional mono-digestion of substrates (such as animal waste) is not appropriate since ammonia toxicity from the rapid degradation of organic nitrogen (such as urea and protein) can result in digester instability [27, 29•]. Recently, a significant opportunity, specifically the anaerobic co-digestion (ACoD) of lignocellulose with animal manure (or other nitrogen-rich organic wastes), was explored to prevent the aforementioned problem and adverse environmental impacts caused by commercial AD facilities (e.g., GHG emission and phytotoxicity). The co-digestion of carbohydrate-rich lignocellulosic biomass with nitrogen-rich animal waste has significant implications in maintaining an optimal C/N ratio for commercial bio-chemicals production [27].
Typically, the residence time of the substrates using ACoD is shorter than that of conventional AD to prevent the digestion of cellulose [29•]. The consortium of microorganisms present during ACoD can convert the sugar constituents of hemicellulose into CH4, while effectively exposing lignin and cellulosic fibers in the digestate. In addition, the solubilized components in the digestate can be used as precursors for diverse products such as bioenergy/biofuel (i.e., CH4, H2, ethanol, and butanol), organic acids (e.g., succinic acid), and bio-polymers (e.g., bioplastic), or applied to agricultural land as an organic fertilizer to promote nutrient retention [51, 52]. It was found that the digestates can be potentially utilized as fertilizer in agriculture due to their contents of N, P, K, and micronutrients [51]. Fuchs and Drosg [52] evaluated the state-of-the-art processing technologies for digestates, which indicate that, in many cases, direct land application is still the most economical treatment option.
Integrated Bio-conversion and Separations Process
One of the main challenges to deploying bio-based chemical technologies is the high cost of product recovery (purification) from the bioreactor due to low product titers [12]. Part of the reasons is that a high (organic) acid concentration inhibits microbial activity, which also results in the reduction of the productivity. Another reason may be due to the low feedstock concentration if it is generated from the lignocellulose biomass. To overcome these technological barriers, different strategies, such as (1) selection of the best pretreatment technology for each feedstock, (2) detoxification of hydrolysate, (3) adaptation and optimization of microorganisms to inhibition, and (4) in situ reaction/separation, have been proposed. For example, since there are no indications that one pretreatment method will be the best route for all biomass feedstocks, it would be possible to achieve a higher yield/concentration of substrate, thereby reducing production cost via selection of the best pretreatment process for different target feedstock. Meanwhile, detoxification of hydrolysate is doable to improve the efficiency of fermentation but it is expensive [41•]. Another option would be adaptation of microorganisms to the inhibitory compounds, such as engineered/adapted strains and cell immobilization or encapsulation.
Integrating the bio-conversion and in situ product recovery is a new approach to cost-competitive bio-chemicals production. The concept of in situ product separation during bio-conversion is the principle of separative bioreactor (SB), where two types of design modulus were commonly used: (1) single-stage and (2) two-stage, or so-called integrated process. The detailed description can be found in the literature [12, 53, 54]. The SB results in a simpler bioprocess train with fewer unit operations, better pH control, reduced inhibitory organic acid compounds, higher organic acid purity, and most importantly, enhanced bio-conversion rates and yields.
Nanofiltration (NF) membranes can reject small molecules such as multivalent ions and monosaccharides, while permeating monovalent ions. Xiong et al. [31] used two commercially available NF membranes to efficiently separate a mixture of carboxylic acids from acidogenic digestion while simultaneously retaining sugars in actual lignocellulosic biomass digestion liquor. The process achieved separation by high sugar rejection (>90 %) and low acid rejection (0–40 %), with the exception of butyric acid (100 % rejection).
Lin et al. [12] has developed an integrated fermentation SB for bio-based chemical production by incorporating bio-conversion process with innovative membrane separations (i.e., resin-wafer electrodeionization, RW-EDI). Cost-competitive bio-based chemical production such as succinic acid, gluconic acid, and sorbitol has been reported using this platform. For example, ∼48 wt% succinic acid is achievable in the anaerobic fermentation using RW-EDI-based SB, with a fermentation productivity and product purity of 0.25–0.30 g/L/h and 80 %, respectively. It combines 1 mol of CO2 with 1 mol of glucose to produce 1 mol of succinic acid, with other byproducts, which implicates the potency to integrate CO2 capture process with the RW-EDI/SB for bio-based chemical production. On the other hand, according to the experience of a pilot-scale test for continuous operation of more than 600 h, the gluconic acid can be produced via aerobic fermentation SB at a productivity of 20–22.5 g/L/h. In addition, the fermentation productivity increased significantly at the pilot scale (in comparison to bench scale ∼5 g/L/h). It was noted that the economic viability of using SB to produce and recover organic acids was in the range of $0.10–0.35 per kg of organic acid (exclusive of labor).
Conclusions
Over the past few decades, the interest in the development of cost-effective and environmentally friendly biotechnological processes using lignocellulosic substrates has increased considerably. Pretreatment of lignocellulose substrates is a critical stage in production to improve the transformation yields and rates because it can break down the lignin structure, disrupt the crystalline structure of cellulose, and increase the porosity of the biomass. Novel pretreatment processes such as microwaves, supercritical fluid extraction, and oxidation methods have been proposed and demonstrated. Beside pretreatment, there is significant development of simultaneous bio-conversion and separations processes such as applying electrochemical methods and/or integrated membrane processes. For example, the innovative membrane technology, i.e., resin-wafer electrodeionization (RW-EDI), with a separative bioreactor (SB) enables continuous product formation and recovery of organic acids while avoiding product inhibition. In addition, the SB is capable of producing organic acids without the needs of neutralization and subsequent acid regeneration, thereby significantly reducing the production cost and environmental impact. Compared to conventional fermentation processes, the SB processes show excellent productivity and high titers of bio-based chemicals.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Adom F, Dunn JB, Han J, Sather N. Life-cycle fossil energy consumption and greenhouse gas emissions of bioderived chemicals and their conventional counterparts. Environ Sci Technol. 2014;48(24):14624–31.
Tsiropoulos I, Faaij APC, Lundquist L, Schenker U, Briois JF, Patel MK. Life cycle impact assessment of bio-based plastics from sugarcane ethanol. J Clean Prod. 2015;90:114–27.
OECD. The Bioeconomy to 2030: designing a policy agenda. Organization for Economic Co-operation and Development; 2009.
Golden JS, Handfield RB. Why biobased? Opportunities in the emerging bioeconomy. Washington: U. S. Department of Agriculture; 2014. p. 40.
de Jong E, Higson A, Walsh P, Wellisch M. Bio-based chemicals: value added products from biorefineries. IEA Bioenergy: Task 42 Biorefinery; 2012.
Chen X, Zhou L, Tian K, Kumar A, Singh S, Prior BA, et al. Metabolic engineering of Escherichia coli: a sustainable industrial platform for bio-based chemical production. Biotechnol Adv. 2013;31(8):1200–23.
Logan BE, Rabaey K. Conversion of wastes into bioelectricity and chemicals by using microbial electrochemical technologies. Science. 2012;337:686–90.
Budarin VL, Shuttleworth PS, De bruyn M, Farmer TJ, Gronnow MJ, Pfaltzgraff L, et al. The potential of microwave technology for the recovery, synthesis and manufacturing of chemicals from bio-wastes. Catal Today. 2015;239:80–9.
Tibbetts SM, Bjornsson WJ, McGinn PJ. Biochemical composition and amino acid profiles of Nannochloropsis granulata algal biomass before and after supercritical fluid CO2 extraction at two processing temperatures. Anim Feed Sci Technol. 2015;204:62–71.
Yen HW, Yang SC, Chen CH, Jesisca CJS. Supercritical fluid extraction of valuable compounds from microalgal biomass. Bioresour Technol. 2015;184:291–6.
Jung YH, Kim HK, Park HM, Park YC, Park K, Seo JH, et al. Mimicking the Fenton reaction-induced wood decay by fungi for pretreatment of lignocellulose. Bioresour Technol. 2015;179:467–72.
Lin YJ, Hestekin JA, Henry MH, Sather N. Bioprocessing of cost-competitive biobased organic acid. In: Snyder SW, editor. Commercializing biobased products. 2015.
Albuquerque TLD, da Silva IJ, de Macedo GR, Rocha MVP. Biotechnological production of xylitol from lignocellulosic wastes: a review. Process Biochem. 2014;49(11):1779–89.
Wilson K, Lee AF. Bio-based chemicals from biorefining: carbohydrate conversion and utilisation. Advances in Biorefineries. Woodhead. 2014. pp. 624–658.
Anwar Z, Gulfraz M, Irshad M. Agro-industrial lignocellulosic biomass a key to unlock the future bio-energy: a brief review. J Radiat Res Appl Sci. 2014;7(2):163–73.
de Jong E, Gosselink RJA. Lignocellulose-based chemical products. Bioenergy research: advances and applications. Elsevier B.V. 2014. pp. 277–313. This book provides an overview of the most important pretreatment and fractionation technologies for lignocellulosic biomass, and their effect on subsequent fermentative/chemocatalytic conversions.
Su H, Liu G, He M, Tan F. A biorefining process: sequential, combinational lignocellulose pretreatment procedure for improving biobutanol production from sugarcane bagasse. Bioresour Technol. 2015;187:149–60.
Martins LH, Rabelo SC, Costa AC. Effects of the pretreatment method on high solids enzymatic hydrolysis and ethanol fermentation of the cellulosic fraction of sugarcane bagasse. Bioresour Technol. 2015;191:312–21.
Xue J, Yang Z, Han L, Liu Y, Liu Y, Zhou C. On-line measurement of proximates and lignocellulose components of corn stover using NIRS. Appl Energy. 2015;137:18–25.
Tippkotter N, Duwe AM, Wiesen S, Sieker T, Ulber R. Enzymatic hydrolysis of beech wood lignocellulose at high solid contents and its utilization as substrate for the production of biobutanol and dicarboxylic acids. Bioresour Technol. 2014;167:447–55.
Ndaba B, Chiyanzu I, Marx S, Obiero G. Effect of Saccharomyces cerevisiae and Zymomonas mobilis on the co-fermentation of sweet sorghum bagasse hydrolysates pretreated under varying conditions. Biomass Bioenergy. 2014;71:350–6.
Su Y, Du R, Guo H, Cao M, Wu Q, Su R, et al. Fractional pretreatment of lignocellulose by alkaline hydrogen peroxide: characterization of its major components. Food Bioprod Process. 2015;94:322–30.
Xu J, Wang X, Hu L, Xia J, Wu Z, Xu N, et al. A novel ionic liquid-tolerant Fusarium oxysporum BN secreting ionic liquid-stable cellulase: consolidated bioprocessing of pretreated lignocellulose containing residual ionic liquid. Bioresour Technol. 2015;181:18–25.
Chiou BS, Valenzuela-Medina D, Bilbao-Sainz C, Klamczynski AK, Avena-Bustillos RJ, Milczarek RR, et al. Torrefaction of pomaces and nut shells. Bioresour Technol. 2015;177:58–65.
Cheirsilp B, Kitcha S. Solid state fermentation by cellulolytic oleaginous fungi for direct conversion of lignocellulosic biomass into lipids: fed-batch and repeated-batch fermentations. Ind Crop Prod. 2015;66:73–80.
Motte J-C, Sambusiti C, Dumas C, Barakat A. Combination of dry dark fermentation and mechanical pretreatment for lignocellulosic deconstruction: an innovative strategy for biofuels and volatile fatty acids recovery. Appl Energy. 2015;147:67–73.
Giuliano A, Bolzonella D, Pavan P, Cavinato C, Cecchi F. Co-digestion of livestock effluents, energy crops and agro-waste: feeding and process optimization in mesophilic and thermophilic conditions. Bioresour Technol. 2013;128:612–8.
Zhang J, Geng A, Yao C, Lu Y, Li Q. Xylitol production from D-xylose and horticultural waste hemicellulosic hydrolysate by a new isolate of Candida athensensis SB18. Bioresour Technol. 2012;105:134–41.
Sawatdeenarunat C, Surendra KC, Takara D, Oechsner H, Khanal SK. Anaerobic digestion of lignocellulosic biomass: challenges and opportunities. Bioresour Technol. 2015;178:178–86. This review paper highlights the current status, challenges, and future research needs of lignocellulosic biomass digestion.
Lammens TM, Franssen MCR, Scott EL, Sanders JPM. Availability of protein-derived amino acids as feedstock for the production of bio-based chemicals. Biomass Bioenergy. 2012;44:168–81.
Xiong B, Richard TL, Kumar M. Integrated acidogenic digestion and carboxylic acid separation by nanofiltration membranes for the lignocellulosic carboxylate platform. J Membr Sci. 2015;489:275–83.
Antonio M, Bünger M, Lee B, Soare A, Yu Y-S. Cultivating capacity for bio-based materials and chemicals through 2017. Lux Research. 2013.
Hackett M. Bio-based chemicals receive boost from shale gas boom in North America. IHS Press; 2014.
Arora MB, Hestekin JA, Snyder SW, St Martin EJ, Lin YJ, Donnelly MI, et al. The separative bioreactor: a continuous separation process for the simultaneous production and direct capture of organic acids. Sep Sci Technol. 2007;42(11):2519–38.
Ghaffar T, Irshad M, Anwar Z, Aqil T, Zulifqar Z, Tariq A, et al. Recent trends in lactic acid biotechnology: a brief review on production to purification. J Radiat Res Appl Sci. 2014;7(2):222–9.
Breeden SW, Clark JH, Farmer TJ, Macquarrie DJ, Meimoun JS, Nonne Y, et al. Microwave heating for rapid conversion of sugars and polysaccharides to 5-chloromethyl furfural. Green Chem. 2013;15(1):72–5.
Crampon C, Mouahid A, Toudji S-AA, Lépine O, Badens E. Influence of pretreatment on supercritical CO2 extraction from Nannochloropsis oculata. J Supercrit Fluids. 2013;79:337–44.
Zhou L, Niu DD, Tian KM, Chen XZ, Prior BA, Shen W, et al. Genetically switched D-lactate production in Escherichia coli. Metab Eng. 2012;14(5):560–8.
Park SJ, Lee TW, Lim SC, Kim TW, Lee H, Kim MK, et al. Biosynthesis of polyhydroxyalkanoates containing 2-hydroxybutyrate from unrelated carbon source by metabolically engineered Escherichia coli. Appl Microbiol Biotechnol. 2012;93(1):273–83.
Kang Y, Bansal P, Realff MJ, Bommarius AS. SO2-catalyzed steam explosion: the effects of different severity on digestibility, accessibility, and crystallinity of lignocellulosic biomass. Biotechnol Prog. 2013;29(4):909–16.
Behera S, Arora R, Nandhagopal N, Kumar S. Importance of chemical pretreatment for bioconversion of lignocellulosic biomass. Renew Sust Energ Rev. 2014;36:91–106. This paper demonstrates various chemical pretreatment processes and their feasibility of the processes at industrial scale, in terms of the mechanisms, advantages, disadvantages and economic assessment.
Davis R, Tao L, Tan ECD, Biddy MJ, Beckham GT, Scarlata C, et al. Process design and economics for the conversion of lignocellulosic biomass to hydrocarbons: dilute-acid and enzymatic deconstruction of biomass to sugars. National Renewable Energy Laboratory (NREL). 2013.
Chu Q, Li X, Yang D, Xu Y, Ouyang J, Yu S, et al. Corn stover bioconversion by green liquor pretreatment and a selected liquid fermentation strategy. BioResource. 2014;9(4):7681–95.
Wu C, Budarin VL, Gronnow MJ, De Bruyn M, Onwudili JA, Clark JH, et al. Conventional and microwave-assisted pyrolysis of biomass under different heating rates. J Anal Appl Pyrolysis. 2014;107:276–83.
Kornarov VV. Handbook of dielectric and thermal properties of materials at microwave frequencies. Artech House. 2012.
Mushtaq F, Mat R, Ani FN. A review on microwave assisted pyrolysis of coal and biomass for fuel production. Renew Sust Energ Rev. 2014;39:555–74.
Sanders JPM, Clark JH, Harmsen GJ, Heeres HJ, Heijnen JJ, Kersten SRA, et al. Process intensification in the future production of base chemicals from biomass. Chem Eng Process Process Intensif. 2012;51:117–36.
Huang C-H, Tan C-S. A review: CO2 utilization. Aerosol Air Qual Res. 2014;14:480–99.
Gu T. Pretreatment of lignocellulosic biomass using supercritical carbon dioxide as a green solvent. In: Gu T, editor. Green biomass pretreatment for biofuels production. Netherlands: Springer; 2013. p. 107–25.
Solana M, Rizza CS, Bertucco A. Exploiting microalgae as a source of essential fatty acids by supercritical fluid extraction of lipids: comparison between Scenedesmus obliquus, Chlorella protothecoides and Nannochloropsis salina. J Supercrit Fluids. 2014;92:311–8.
Alburquerque JA, de la Fuente C, Ferrer-Costa A, Carrasco L, Cegarra J, Abad M, et al. Assessment of the fertiliser potential of digestates from farm and agroindustrial residues. Biomass Bioenergy. 2012;40:181–9.
Fuchs W, Drosg B. Assessment of the state of the art of technologies for the processing of digestate residue from anaerobic digesters. Water Sci Technol. 2013;67(9):1984–93.
Datta S, Lin YP, Snyder SW. Current and emerging separations technologies in biorefining. Advances in biorefineries. Cambridge: Woodhead Publishing; 2014. p. 112–51.
Hestekin JA, Lin YP, Frank JR, Snyder SW, Martin EJS. Electrochemical enhancement of glucose oxidase kinetics: gluconic acid production with anion exchange membrane reactor. J Appl Electrochem. 2002;32:1049–52.
Acknowledgments
The submitted manuscript has been created by UChicago Argonne, LLC, Operator of Argonne National Laboratory (“Argonne”). Argonne, a US Department of Energy Office of Science laboratory, is operated under contract no. DE-AC02-06CH11357. The US Government retains for itself, and others acting on its behalf, a paid-up nonexclusive, irrevocable worldwide license in the said article to reproduce, prepare derivative works, distribute copies to the public, and perform publicly and display publicly, by or on behalf of the government. In addition, high appreciation goes to the Ministry of Science and Technology (MOST) of Taiwan (Republic of China) under Grant Number MOST 103-2911-I-002-596 for the financial support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Sustainable and Renewable Fuels
Rights and permissions
About this article
Cite this article
Pan, SY., Lin, Y.J., Snyder, S.W. et al. Development of Low-Carbon-Driven Bio-product Technology Using Lignocellulosic Substrates from Agriculture: Challenges and Perspectives. Curr Sustainable Renewable Energy Rep 2, 145–154 (2015). https://doi.org/10.1007/s40518-015-0040-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40518-015-0040-y