Abstract
Biopolymer “Chitosan” has received much interest for potential wide application in agriculture due to its excellent biocompatibility, biodegradability and bioactivity. This naturally occurring molecule with interesting physiological potential has been getting more attention in recent years. Chitosan enhanced the efficacy of plants to reduce the deleterious effect of unfavorable conditions as well as on plant growth. Chitosan affects various physiological responses like plant immunity, defense mechanisms involving various enzymes such as, phenylalanine ammonium lyase, polyphenol oxidase, tyrosine ammonia lyase and antioxidant enzymes viz., activities superoxide dismutase, catalase and peroxide against adverse conditions. Recent studies have shown that chitosan induces mechanisms in plants against various biotic (fungi, bacteria, and insects) and abiotic (salinity, drought, heavy metal and cold) stresses and helps in formation of barriers that enhances plant's productivity. This paper takes a closer look at the physiological responses of chitosan molecule.
Similar content being viewed by others
Introduction
Application of new technologies have resulted in rapid advances in the agriculture, and made it possible to achieve target of crop production. However, for continuation of advances in agricultural productivity, more environmental friendly production technologies must be followed. Some environmental-friendly products that have been widely used in agricultural applications are for stimulation of plant defenses (Yu and Meuhlbauer 2001).
Polysaccharide chitosan, a linear polysaccharide composed of randomly distributed β-(1-4)-linked d-glucosamine (deacetylated unit) and N-acetyl-d-glucosamine (acetylated unit) is an environmental friendly product. Chitin can be easily obtained from shellfish waste. The chitosan molecule triggers defense responses within the plant, leading to the formation of physical and chemical barriers against invading pathogens. Chitosan has been used to stimulate the immunity of plants to protect plants against microorganisms. This protection leads stimulation of plant growth (Bautista et al. 2003).
The present review, explores the positive effect of chitosan on the growth of roots, shoots and leaves of various plants. Chitosan oligomer "Oligochitosan" has shown a wide range of biological applications, including health, food, plant growth stimulator, feed additive, antimicrobial activity, etc. In addition, oligochitosan is effective at eliciting plant innate immunity against plant diseases in tobacco, rapeseed, rice, grapevine (Agrawal et al. 2002; Chen et al. 2009; Rakwal et al. 2002). Oligochitosan can be produced in laboratory by enzymatic hydrolysis. It has a potent protective effect on plants such as grain crops, fruits, vegetables and trees (Zhao et al. 2009).
The aim of this review is to collate, summarize and critically appraise the published evidence on the physiological responses of chitosan and assess the existing beneficial responses on plant immunity, defense mechanisms, germination, plant growth, photosynthesis, heavy metals stresses etc.
Chitosan and its characteristics
Chitin, a homo polymer comprising β-(1-4)-linked N-acetyl-d-glucosamine residues, is one of the most abundant, easily obtainable and renewable natural polymers, second only to cellulose. Chitin derivative, chitosan is a poly (1, 4)-2-amino-2-deoxy-β-d glucose. It is a copolymer of 2-glucosamine and N-acetyl-2-glucosamine, derived from chitin, by deacetylation reaction (Fig. 1). The d-glucosamine content in chitosan is indicated by the degree of deacetylation (DDA) (Hejazi and Amiji 2003). Chitosan is a polymer of high molecular weight, similar to cellulose. The only difference between chitosan and cellulose is the amine (–NH2) group in the position C-2 of chitosan instead of the hydroxyl (–OH) group found in cellulose. However, unlike plant fiber, chitosan possesses positive ionic charges, which gives it the ability to chemically bind with negatively charged lipids, metal ions, proteins, and macromolecules (Li et al. 1992).
Chemical structure of chitosan (George and Abraham 2006)
Chitosan has been widely applied in functional foods, food additives, environmental protection and biotechnology (Shahidi et al. 1999). Moreover, various studies have shown that chitosan has antifungal and antimicrobial effects (Kumar et al. 2004). Chitosan and its derivatives have shown various functional properties, which made it possible to be used in many fields including, food, cosmetics (Majeti and Kumar 2000), biomedicine (Felt et al. 1998), agriculture (Yamada et al. 1993), environmental protection and wastewater management (Kamble et al. 2007). Further, biodegradable, non-toxic and non-allergenic natures of chitosan encourage its potential use as a bioactive material (Kurita 1998; Katiyar et al. 2011).
Application of chitosan in agriculture
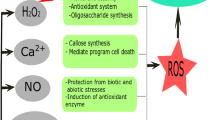
The unique physiological and biological properties of chitosan have led to its use in various industries, in including agriculture, as a coating material for fruits, seeds and vegetables (Lee et al. 2005). Chitosan stimulate plant immune systems, protect plants against attack by microorganisms, growth and crop productivity (Fig. 2).
Effect of oligochitosan on plant immunity
Unlike animals, plants are sessile and therefore, these have developed sophisticated mechanisms to adapt to various biotic (fungi, bacteria, and insects) and abiotic (wounding, salinity, drought, salt, and cold) stresses. To resist these stresses, plants have evolved the ability to initiate various defense reactions such as hypersensitive responses, production of phytoalexins, and reinforcement of cell walls etc. The immunity stimulating activity of oligochitosan have been documented in many different plant systems. The potent effect of chitosan on plant diseases is due to its antimicrobial properties and plant innate immunity elicitation activity. The antimicrobial activity is influenced by several factors, such as molecular weight, DDA, solubility, positive charge density, chemical modification, pH, concentration, chelating capacity and type of microorganism. Chitosan has also used as a promising postharvest treatment for fruits due to its natural character, antimicrobial activity and elicitation of defense responses (Katiyar et al. 2014). Chitosan has been used to control postharvest diseases of many fruits such as pear (Yu et al. 2008), strawberry (Ge et al. 2010), table grape (Meng et al. 2008), tomato (Badawya and Rabeab 2009), citrus and longan (Jiang and Li 2001).
Chitosan is one of the most important elicitors. Researchers have proved that it elicit plant defense response to a broad spectrum of phytopathogens, including plant virus (Terry and Joyce 2004). At present many experimental results have proved that chitosan can inhibit viral infection (Pospieszny 1997). Treatment of bean with chitosan decreased the number of local necroses caused by alfalfa mosaic virus (AMV) infection. It has been shown that chitosan inhibited the infection caused by the bacteriophage, the efficiency of inhibition of bacteriophage infection depends directly on the final concentration in the medium (Ma et al. 2008). Major factors for suppressing phage infection by chitosan are phage particle's inactivation and inhibition of reproduction at the cellular level. Evidently, chitosan may be used for induction of phago resistance in industrial microorganism cultures to prevent undesirable phagolysis caused by inoculum contamination by virulent bacteriophages or by spontaneous prophage induction in lysogenic cultures.
These studies have shown that chitosan treatment in plants could suppress virus infections regardless of virus types as well as plant species. However, the antiviral activity mainly depends on the molecular structure of chitosan, especially on the molecular weight. It was found that low molecular-weight chitosan (oligochitosan) is more effective in suppressing infection of the tobacco mosaic virus (TMV) in tobacco plants, and antiviral activity of chitosan increased as its molecular weight decreased (Kulikov et al. 2006). The efficient antiviral activity of low molecular weight fractions is presumably due to its better penetrability across the integuments of seeds (Kulikov et al. 2006). Although chitosan has been proved to be effective in plant resistance against plant virus, the mechanism still remains obscure. It has been reported that chitosan activated systemic acquired resistance against tobacco necrosis virus (TNV) ensued from a programmed cell death, which was similar to that occurred in the hypersensitive response (Iriti et al. 2006).
Chitosan application can also elicit callose deposition, which has a partial effect on inhibiting virus spreading (Iriti and Faoro, 2008). The activation of a Ca2+-dependent callose synthase is one of the most rapid, effective cell responses to chitosan treatment. Calcium is one of the most important second messenger in innumerous plant signaling pathways. In recent years pathogen elicitors have been shown to induce changes in cytosolic free calcium concentration ([Ca2+]cyt) (White and Broadley 2003). Many plant defense responses are mediated by changes of [Ca2+]cyt (Hamel et al. 2005). In tobacco, laminarin sulfate induced the resistance against TMV infection after Ca2+ dependent oxidative burst (Menard et al. 2004). Lipopolysaccharides are able to promote plant disease tolerance through activation of Ca2+ signal (Gerber et al. 2004). Chitosan, which has been shown to induce elevation of [Ca2+] cyt, activates plant defense responses through the calcium signaling pathway (Klusenser et al. 2002). So Ca2+ is one of the early defense responses against pathogen infection.
Role of chitosan in defense mechanisms
Chitosan has been demonstrated to induce defense mechanism in tomato, cucumber (Ben et al. 2003), and rose shrubs (Wojdyla 2004). Several studies have shown that chitosan stimulates other systems like transduction, cascades and elicitor-responsive genes involved in resistance of plants to infection (Vander et al. 1998; Kim et al. 2005). Chitosan induces the accumulation of phytoalexins resulting in antifungal responses and enhanced protection from further infections (Vasyukova et al. 2001). Spraying with chitosan has been shown to significantly reduce severity of leaf spot disease and increase the length of inflorescences in Dendrobium Missteen.
Chitosan treatment increased polyphenol oxidase (PPO) activity in disease resistant cultivars (Raj et al. 2006).Oxidation of phenolic compounds associated with enhanced resistance to pathogens may involve PPO, which could generate reactive oxygen species (ROS) (Mayer 2006). Kim et al. (2005) reported that in sweet basil chitosan and methyl jasmonate increased antioxidant activity of 2,2-diphenyl-1-picrylhydrazyl (DPPH) by 3.5- and 2.3-folds, respectively. Pre incubation of suspension-cultured wheat cells in a growth medium of Pantoeaag glomerans with chitin or chitosan led to a strong increase in extracellular peroxidase activity (Ortmann and Moerschbacher 2006).
Chitosan has been reported to enhance phytoalexin production in germinating peanut and in legumes and solanaceous plants (Cote and Hahn 1994). Chitosan may be involved in the signaling pathway for the biosynthesis of phenolics. It has been shown that chitosan can induce chitinase and chitosanase, which are members of a group of plant pathogenesis related (PR) proteins. These PR proteins can degrade the cell walls of some phyto pathogens and consequently may play a role in host plant defense systems (Dixon et al. 1994). Moreover, chitosan can also induce plant immune systems (systemic acquired resistance or SAR), which is long lasting and often confers broad-based resistance against different pathogens. SAR can develop in uninfected parts of the plant, as a result the entire plant becomes more resistant to a secondary infection. Chitosan has been shown to elicit defense genes in several species such as rice (Rakwal et al. 2002). Further studies have shown that chitosan induces the expression of various genes involved in plant defence responses such as genes encoding PAL and protease inhibitors (Vander et al. 1998). Genetic studies have shown that chitosan may involve jasmonic acid (JA) pathways, since transcription activation of genes encoding PAL and protease inhibitors are induced by both JA and chitosan (Farmer and Ryan 1992). Chitosan probably could alleviate the membrane lipid peroxidization and decrease phytotoxicities in plant cells,which can reduce plant cell stress caused by high chemical oxygen demand (COD) in polluted water (Xu et al. 2007). Therefore, the antifungal action of chitosan seems to comprise more than one mode of action by which chitosan affects fungal cell wall biosynthesis and/or alteration of the ability of pathogens to infect and/or its ability to increase plant resistance.
Chitosan as plant defense booster
Chitosan has been described as “plant defense booster”. The term ‘plant defense booster’ applies to a group of compounds, which act by triggering various physiological and morphological responses within the plant that help to stimulate natural defense mechanisms. The practical significance of plant defense boosters is that they can help to reduce the amount of crop protection chemicals applied to crops. Results from the literature indicate variability in plant responses following application of plant defense boosters such as chitosan. Quang et al. (2006) reported that positive effects of chitosan on barely plants depend on the molecular weight of the applied chitosan. Oligochitosan application excites a series of defense responses in rice to enhance the disease resistance to Magnap or the Grisea (Ning et al. 2003).
Chitosan biosynthesis in fungi (Hadwiger 2009) starts with the sugar nucleotide, uridine diphosphate N-acetyl-d-glucosamine (UDP-GlcNAc) that is incorporated into chitin.(Fig. 3) Two enzymes, chitin synthetase and chitin deacetylase produce chitosan (Kafetzopoulos et al. 1993). Chitinase recognizes β-1, 4-linked N-acetyl glucosamine residues, cleaving these regions within chitosan producing oligomers. The commercial production of chitosan originates as crustacean chitin. Chitosan oligomers can be derived from HCl or enzymatic digestion of chitosan (Cabrera and Cutsem 2005). Some plants have lectin-like proteins that complex with chitosan and are candidate receptors (Chen and Xu 2005). Protein kinase cascades that may relay the signal to transcription factors (TRs) have not been identified. Chitosan as a polycationic polymer elicits cellular changes (Yin et al. 2010), viz, membrane depolarization, oxidative burst, influx and exit of ions such as Ca2+, kinases, DNA alteration, mRNA, PR proteins, phytoalexins, lignifications and callose deposition. A proposed direct elicitation of gene activity in plant defense implicates both chitosan/DNA interactions and fungal DNase/DNA interactions (Hadwiger 2008). Direct effects of chitosan oligomers on defense gene transmission have been proposed to occur by (a) alterations of DNA helical structure, single strand cleavage and removal of histones H2A and H2B, along with reductions in the architectural transcription factor, HMG A (Weake and Workman 2008), (b) chitosan may compete with histones for sensitive DNA sites allowing stalled DNA polymerase complexes to continue to transcribe through the open reading frames of PR genes (Schwabish and Struhl 2004). The fungal derived elicitors, chitosan oligomers and DNase, are capable of both inducing PR gene products and inhibiting fungal growth. Signal peptides on some PR proteins enable transit from the plant cell to the germinating spore (Benhamou et al.1994).
Enhancement of antioxidant enzyme activities by chitosan
Reactive oxygen species (ROS) are related to light dependent events produced in plants even under optimal conditions. Therefore, photosynthetic cells are easily damaged by oxidative stress, which injures the cellular components like, proteins, nucleic acids and membrane lipids (Foyer et al. 1994). It has been reported that oligochitosan induces accumulation of H2O2 in plants (Lin et al. 2005). It is involved in the oxidative burst and the induction of the ROS scavenging system (Agrawal et al. 2002). Enzymatic systems consisting of superoxide dismutase (SOD), catalase (CAT) and peroxidase (POX) are responsible for scavenging of these ROS in plants (Ahmad et al. 2013). Guo et al. (2003) reported induction of SOD and CAT by oligochitosan treatment in leaves. It was shown that POX activity increased by 0.0625 and 0.125 % by oligochitosan in wheat leaves compared with control. Thus, increased CAT and POX activities helped in wheat seedling development (Ma et al. 2014). Yin et al. (2008) reported that oligochitosan upregulated the activities of PAL, PPO, POX, CAT and SOD in tobacco and Brassica napus.
Chitosan improves the germination
Ma et al. (2014) reported that oligochitosan promoted wheat growth in terms of germination capacity, root length, seedling height and increase in root activity. Batool and Asghar (2013) reported that seeds of Carum copticum primed with different concentrations of chitosan showed increase in germination percentage, germination rate, seedling vigor index, length and dry weight of hypocotyl and radical compared to control. Shao et al. (2005) reported that seed soaked with chitosan increased the germination percentage of maize seed. Manjunatha et al. (2008) reported that seed priming with chitosan enhanced seed germination and seedling vigour in pearl millet. Seed soaked with chitosan increased germination rate, length and weight of hypocotyls and radical in rapeseed (Sui et al. 2002). Similarly, No et al. (2003) observed that treatment with 493 kDa chitosan improved growth in soybean sprout. Chitosan treatment increased total weight as well as the length and thickness of sunflower hypocotyls compared with control (Cho et al. 2008).
Zeng and Luo (2012) reported that chitosan has excellent film forming property, making it easy to form a semi permeable film on the seed surface, which can maintain the seed moisture and absorb the soil moisture, and thus it can promote seed germination. Priming with 0.2 % chitosan was found to be superior to all other concentrations. Researcher have reported that chitosan increased shoot length, root length, shoot dry weight and relative water content under salinity stress. Similar results were reported by Ma et al. (2012), who reported that wheat seeds treated with chitosan showed higher growth than control under salinity stress. Cote and Hahn (1994) suggested that the oligosaccharides act on plants in a manner similar to phytohormone in regulation of morphogenesis, growth and development. It is suggested that chitosan triggers the defensive mechanisms in plants, stimulates root growth and induces certain enzymes such as chitinases, pectinases and glucanases (Hien 2004). Chitosan promotes plant growth through increasing the availability and uptake of water and essential nutrients through adjusting cell osmotic pressure (Guan et al. 2009).
Chitosan in drought tolerance
The content of leaf water reflects the water status of plant. Chitosan coating can improve the leaf water content of seedlings. The experimental results showed that chitosan significantly increased the concentration of chlorophyll under drought stress, which illustrates that chitosan can enhance the photosynthesis performance and the accumulation of organic matter in wheat seedlings. Under the drought condition, a well developed root system absorbs more water to keep the moisture stable (Zhang et al. 2002). Chitosan coating can reduce the inhibition of roots and stem growth under drought stress, which shows chitosan can effectively promotes the development of root system and strengthens the capability of water absorption, so as to enhance drought resistance of wheat seedlings (Zeng and Luo 2012).
Oligochitosan application reduced the decline in photosynthetic rate in B. napus seedlings under drought stress (Li et al.2008), and ameliorated the adverse effects of salt stress in wheat seedlings (Ma et al. 2012). Therefore, oligochitosan has a strong potential application value in agriculture. Electron microscopy and histochemical analyses have demonstrated that foliar application of chitosan reduced transpiration of pepper plants through partial or full closure of stomata (Bittelli et al. 2001). Chitosan was found to reduce plant transpiration in pepper plants resulting in a 26–43% reduction in water use, while maintaining biomass production and yield. These results suggested that chitosan might be an effective anti transpirant to conserve water use in agriculture (Bittelli et al. 2001).
Effect of chitosan on plant growth
Chitosan also promoted growth of various crops such as cabbage (Brassicaoleracea L.) Hirano 1988), soybean sprouts (Lee et al. 2005) and sweet basil (Kim et al. 2005). Chitogel, a derivative of chitosan, was found to improve vegetative growth of grapevine plantlets (Barka et al. 2004). This study showed that the average O2 production by plantlets cultured on medium supplemented with 1.75 % chitogel increased 2-fold, whereas CO2 fixation increased only 1.5-fold, indicating that chitogel had a beneficial effect on net photosynthesis in plantlets and confirmed its positive effects on grapevine physiology. It has also been shown that chitosan promotes vegetative growth and enhances various processes in developing flower buds (Barka et al. 2004). N-acetyl chitosaccharides concentration was varied in growth media depending on the chemo/ physiological conditions of the cells, which caused much higher root growth than in control production media. Chitosan treated orchid plants (Dendrobium Sensational ‘Purple’) had more flower shoots and yields tended to be higher compared to the control plants (Chandrkrachang et al. 2005).
Chitosan enhances the production of secondary metabolites
The use of biotic or abiotic elicitors is one way to increase the yields of secondary metabolites in in vitro cultures. Supplementation of hairy root cultures of Brugmansia candida with chitosan at certain concentrations was found to increase the content of root scopolamine and hyoscyamine, which are valuable anticholinergic drugs employed as antispasmodics and in the treatment of motion sickness. Both are members of the tropane group of alkaloids (Hashimoto et al. 1993). Hairy root cultures of Hyoscyamus muticus supplemented with chitosan produced 5-fold more hyoscyamine than the control (Sevon et al. 1992). Treating hairy root cultures of Trigonella foenum-graecum L. with 40 mg l−1 chitosan induced a threefold increase in diosgenin, α spirostanol, important for the synthesis of steroid hormones (Merkli et al.1997).
Chitosan enhanced storage life and quality of fruits
Chitosan can be used as a coating material for fruit (Jiang and Li 2001). Results of some previous studies have shown that chitosan coating has the potential to prolong storage life and to control decay of many fruit such as strawberries (Hernandez et al. 2006), and papaya (Sivakumar et al. 2005). Chitosan treatment have been reported to prolongs storage life and controls decay of cucumber, carrot, apple, citrus, kiwifruit, peach, pear, strawberry, and sweet cherry (Ben et al. 2003). One of the unique characteristics of chitosan-based coating is that it is a carrier for incorporating functional ingredients, such as antimicrobial agents and nutraceuticals (Park and Zhao 2004).
The chitosan based coating can form a protective barrier on the surface of fresh fruit, reduce water loss, inhibit gas exchange, decrease nutrient loss, and prevent micro-organism growth that causes fruit rotting (Qiuping and Wenshui 2007). The application of chitosan combined with ammonium carbonate offers a commercially acceptable, economically viable and effective alternative for postharvest control of anthracnose in stored papaya. Dipping papaya in chitosan plus ammonium carbonate, significantly (P < 0.005) retarded color development of skin and flesh, increased fruit firmness and reduced weight loss (Sivakumar et al. 2005). Chitosan increased the growth of Paphiopedium bellatulum x Paphiopedium angthong in tissue culture. Encapsulating seeds of Spathoglottis plicata with alginate-chitosan has been reported to minimize infections by mycorrhizal fungi (Tan et al. 1998). Researchers have demonstrated that the effectiveness of chitosan depended on molecular weight, ratio of sugar carbons to glucosamine and N-acetyl-glucosamine, and the concentration and frequency of applications.
Miscellaneous effects of chitosan on plants
Increasing levels of abscissic acid (ABA) plays a key role in the regulation of water use by closure of stomata and decreased transpiration in plants. Stomata closure induced by foliar application of chitosan may involve ABA biosynthesis pathways. It has been shown that plant can survive in low water environments by chitosan treatment. Oligochitosan can effectively increased IAA concentration, which promoted the growth of tobacco plants (Guo et al. 2009). Chitosan application at 200 mg kg−1 soil was effective in alleviating the harmful effects of high cadmium concentration on radish plant (Farouk et al. 2011).
Implications for future practice and research
The ubiquity, biological and biocompatible properties of chitosan establishes them as promising bioactive compound for agriculture. Further research is required to validate laboratory results obtained under controlled conditions in India to become potential agricultural practice. Its physiological activities, together with the recently-discovered properties have tremendous utility in agriculture, and could result in development of sustainable agricultural practices, by decreasing the use of synthetic chemical and bringing a new focus to modern plant physiology. These compounds could be of significant use under adverse conditions, such as in low fertility, high salinity, heavy metal contaminated soils, and in soils affected by prolonged drought, under climate change condition. However, all these depend on gaining the focus of researchers, and confidence of farmers and producers of these potential compounds.
Conclusions
Chitosan, derivative of chitin is the second most abundant natural polymer and one of the most widely distributed throughout the nature. The numbers of applications of chitosan and their derivatives have been increasing steadily in the last decade. Chitosan has been shown to be a versatile nontoxic material with multiple responses. To date, there is enough evidence indicating that after chitosan application plants can acquire enhanced tolerance to a wide variety of pathogenic microorganisms, unfavorable climatic conditions and improve growth, indicating that the use of natural elicitors such as chitosan might assist in the goal of sustainable agriculture.
References
Agrawal, G. K., Rakwal, R., Tamogami, S., Yonekura, M., Kubo, A., & Saji, H. (2002). Chitosan activates defense/stress response(s) in the leaves of Oryza sativa seedlings. Plant Physiology and Biochemistry, 40, 1061–1069.
Ahmad, I., Basra, S. M. A., Afzal, I., Farooq, M., & Wahid, A. (2013). Growth improvement in spring maize through exogenous application of ascorbic acid, salicylic acid and hydrogen peroxide. International Journal of Agriculture and Biology, 15, 95–100.
Badawya, M. E. I., & Rabeab, E. I. (2009). Potential of the biopolymer chitosan with different molecular weights to control postharvest gray mold of tomato fruit. Postharvest Biology Technology, 51, 110–117.
Barka, A. E., Eullaffroy, P., Clément, C., & Vernet, G. (2004). Chitosan improves development, and protects Vitis vinifera L. against Botrytis cinerea. Plant Cell Reports, 22, 608–614.
Batool, M., & Asghar, R. (2013). Seed priming with chitosan improves the germination and growth performance of ajowan (Carum copticum) under salt stress. Eurasia Journal of Bioscience, 7, 69–76.
Bautista, B. S., Hernandez, L. M., Bosquez, M. E., & Wilson, C. L. (2003). Effects of chitosan and plant extracts on growth of Colletotrichum gloeosporioides, anthracnose levels and quality of papaya fruit. Crop Protection, 22, 1087–1092.
Ben, B. N., Ardi, R., Pinto, R., Aki, C., & Fallik, E. (2003). Controlling gray mould caused by Botrytis cinerea in cucumber plants by means of chitosan. Crop Protection, 22, 285–290.
Benhamou, N., Lafontaine, L. J., & Nicole, M. (1994). Induction of systemic resistance to Fusarium crown and root rot in tomato plants by seed treatment with chitosan. Phytopathology, 84, 1432–1444.
Bittelli, M. M., Flury, G., Campbell, S., & Nichols, E. J. (2001). Reduction of transpiration through foliar application of chitosan. Agricultural and Forest Meteorology, 107, 167–175.
Cabrera, J. C., & Cutsem, P. V. (2005). Preparation of chitooligosaccharides with degrees of polymerization higher than 6 by acid or enzymatic degradation of chitosan. Biochemical Engineering Journal, 25, 165–172.
Chandrkrachang, S., Sompongchaikul, P., & Sangtain, S. (2005). Profitable spinoff from using chitosan in orchid farming in Thailand. Journal of Metals, Materials and Mineral, 15, 45–48.
Chen, H. P., & Xu, L. L. (2005). Isolation and characterization of a novel chitosan-binding protein from non-heading Chinese cabbage leaves. Journal of Integral Plant Biology, 47, 452–456.
Chen, Y. F., Zhan, Y., Zhao, X. M., Guo, P., An, H. L., Du, Y. G., et al. (2009). Functions of oligochitosan induced protein kinase in tobacco mosaic virus resistance and pathogenesis related proteins in tobacco. Plant Physiology and Biochemisrty, 47, 724–731.
Cho, M. H., No, H. K., & Prinyawiwatkul, W. (2008). Chitosan treatments affect growth and selected quality of sunflower sprouts. Journal of Food Science, 73, 570–577.
Cote, F., & Hahn, M. G. (1994). Oligosaccharin: Structures and signal transduction. Plant Molecular Biolology, 26, 1379–1411.
Dixon, R. A., Harrison, M. J., & Lamb, C. J. (1994). Early events in the activation of plant defenses. Annual Review of Phytopatholgy, 32, 479–510.
Farouk, S., Mosa, A. A., Taha, A. A., Ibrahim, H. M., & EL-Gahmery, A. M. (2011). Protective effect of humic acid and chitosan on radish (Raphanus sativus, L. var. sativus) plants subjected to cadmium stress. Journal of Stress Physiology and Biochemistry, 7, 99–116.
Farmer, E. E., & Ryan, C. A. (1992). Octadecanoid precursors of jasmonic acid activate the synthesis of wound inducible proteinase inhibitors. Plant cell, 4, 129–134.
Felt, O., Buri, P., & Gurny, R. (1998). Chitosan: A unique polysaccharide for drug delivery. Drug Development Industrial Pharmacy, 24, 979–993.
Foyer, C. H., Maud, L., & Kunert, K. J. (1994). Photooxidative stress in plants. Plant Physiology, 92, 696–717.
Ge, L. L., Zhang, H. Y., Chen, K. P., Ma, L. C., & Xu, Z. L. (2010). Effect of chitin on the antagonistic activity of Rhodotorula glutinis against Botrytis cinerea in strawberries and the possible mechanisms involved. Food Chemistry, 120, 490–495.
George, M., & Abraham, T. E. (2006). Polyionic hydrocolloids for the intestinal delivery of protein drugs: alginate and chitosan – a review. Journal of Control Release, 114, 1–14.
Gerber, I. B., Zeidler, D., Durner, J., & Dubery, I. A. (2004). Early perception responses of Nicotiana tabacum cells in response to lipopolysaccharides from Burkholderia cepacia. Planta, 218, 647–657.
Gozzo, F. (2003). Systemic acquired resistance in crop protection: from nature to a chemical approach. Journal of Agricultural and Food Chemistry, 51, 4487–4503.
Guan, Y. J., Hu, J., Wang, X. J., & Shao, C. X. (2009). Seed priming with chitosan improves maize germination and seedling growth in relation to physiological changes under low temperature stress. Journal of Zhejiang University Science B, 10, 427–433.
Guo, H. L., Du, Y. G., Bai, X. F., & Zhao, X. M. (2003). Effects of active oxygen on suspended cotton cell culture by oligochitosan. Chinese Journal of Marine Drugs, 1, 11–12.
Guo, W. H., Ye, Z. Q., Wang, G. L., Zhao, X. M., Yuan, J. L., & Du, Y. G. (2009). Measurement of oligochitosan–tobacco cell interaction by fluorometric method using europium complexes as fluorescence probes. Talanta, 78, 977–982.
Hadwiger, L. A. (2008). Pea–Fusarium solani interactions contributions of a system toward understanding disease resistance. Phytopathology, 98, 372–379.
Hadwiger, L. A. (2009). Localization predictions for gene products involved in non-host resistance responses in a model plant/fungal pathogen interaction. Plant Science, 177, 257–265.
Hadwiger, L. A. (2013). Multiple effects of chitosan on plant systems: Solid science or hype. Plant Science, 208, 42–49.
Haggag, M. W. (2007). Colonization of exopolysaccharide-producing Paenibacillus polymyxa on peanut roots for enhancing resistance against crown rot disease. African Journal of Biotechnology, 6, 1568–1577.
Hamel, L., Miles, G. P., Samuel, M. A., Ellis, B. E., Seguin, A., & Beaudoin, N. (2005). Activation of stress-responsive mitogen-activated protein kinase pathways in hybrid poplar (Populus trichocarpa × Populus deltoides). Tree Physiology, 25, 277–288.
Hashimoto, T., Yun, D. J., & Yamada, Y. (1993). Production of tropane alkaloids in genetically engineered root cultures. Phytochemistry, 32, 713–718.
Hejazi, R., & Amiji, M. (2003). Chitosan-based gastrointestinal delivery systems. Journal of Controlled Release, 89, 151–165.
Hernandez, M. P., Almenar, E., Ocio, M. J., & Gavara, R. (2006). Effect of calcium dipsand chitosan coatings on postharvest life of strawberries (Fragaria x ananass). Postharvest Biology Technology, 39, 247–253.
Hien, Q. N. (2004). Radiation processing of chitosan and some biological effects. Radiation Processing of Polysaccharides, 1, 67–73.
Hirano, S. (1988). The activation of plant cells and their self-defence function against pathogens in connection with chitosan. Nippon Nogeikagaku Kaishi, 62, 293–295.
Iriti, M., & Faoro, F. (2008). Abscisic acid is involved in chitosan-induced resistance to tobacco necrosis virus (TNV). Plant Physiology and Biochemistry, 46, 1106–1111.
Iriti, M., Sironi, M., Gomarasca, S., Casazza, A. P., Soave, C., & Faoro, F. (2006). Cell death- ediated antiviral effect of chitosan in tobacco. Plant Physiology and Biochemistry, 44, 893–900.
Jiang, Y., & Li, Y. (2001). Effect of chitosan coating on postharvest life and quality of longan fruit. Food Chemistry, 73, 139–143.
Kafetzopoulos, D., Martinou, A., & Bouriotis, V. (1993). Bioconversion of chitin to chitosan: Purification and characterization of chitin deacetylase from Mucor rouxii. Proceedings of the National Academy of Sciences of the United States of America, 90, 2564–2568.
Kamble, S. P., Jagtap, S., Labhsetwar, N. K., Thakare, D., Godfrey, S., Devotta, S., & Rayalu, S. S. (2007). Defluoridation of drinking water using chitin, chitosan and lanthanum-modified chitosan. Chemical Engineering Journal, 129, 173–180.
Katiyar, D., Hemantaranjan, A., Singh, B., & Bhanu, N. A. (2014). A future perspective in crop protection: Chitosan and its oligosaccharides. Advances in Plants Agricultural Research, 1, 06.
Katiyar, D., Singh, B., Lall, A. M., & Haldar, C. (2011). Efficacy of chitooligosaccharides for the management of diabetes in alloxan induced mice: A correlative study with antihyperlipidemic and antioxidative activity. European Journal of Pharmaceutical Sciences, 44, 534–543.
Kim, H. J., Chen, F., Wang, X., & Rajapakse, N. C. (2005). Effect of chitosan on the biological properties of sweet basil (Ocimum basilicum L.). Journal of Agricultural and Food Chemistry, 53, 3696–3701.
Klusenser, B., Young, J. J., Murata, Y., Allen, G. J., Mori, I. C., Hugouvieux, V., et al. (2002). Convergence of calcium signaling pathways of pathogenic elicitors and abscisic acid in Arabidopsis guard cells. Plant Physiology, 130, 2152–2163.
Kulikov, S. N., Chirkov, S. N., Il’ina, A. V., Lopatin, S. A., & Varlamov, V. P. (2006). Effect of the molecular weight of chitosan on its antiviral activity in plants. Applied Biochemistry and Microbiology, 42, 200–203.
Kumar, M. N. V. R., Muzzarelli, R. A. A., Muzzarelli, C., Sashiwa, H., & Domb, A. J. (2004). Chitosan chemistry and pharmaceutical perspectives. Chemical Reviews, 104, 6017–6084.
Kurita, K. (1998). Chemistry and application of chitin and chitosan. Polymer Degradation and Stability, 59, 117–120.
Lee, Y. S., Kim, Y. H., & Kim, S. B. (2005). Changes in the respiration, growth, and vitamin C content of soybean sprouts in response to chitosan of different molecular weights. Horticulture Science, 40, 1333–1335.
Li, Q., Dunn, E. T., Grandmaison, E. W., & Goosen, M. F. A. (1992). Applications and properties of chitosan. Journal Bioactive Compatible Polymer, 7, 370–397.
Li, Y., Zhao, X. M., Xia, X. Y., Luan, Y. S., Du, Y. G., & Li, F. L. (2008). Effects of oligochitosan on photosynthetic parameter of Brassica napus seedlings under drought stress. Acta Agronomy Sinica, 34, 326–329.
Lin, W., Hu, X., Zhang, W., Rogers, W. J., & Cai, W. (2005). Hydrogen peroxide mediates defense responses induced by chitosans of different molecular weights in rice. Journal of Plant Physiology, 162, 937–944.
Ma, L. J., Li, Y. Y., Yu, C. M., Wang, Y., Li, X. M., Li, N., et al. (2012). Alleviation of exogenous oligochitosan on wheat seedlings growth under salt stress. Protoplasma, 249, 393–399.
Ma, L. J., Li, Y. Y., Yu, C. M., Wang, Y., Li, X. M., Li, N., et al. (2014). Germination and physiological response of wheat (Triticum aestivum) to pre-soaking with oligochitosan. International Journal of Agricultural Biology, 16, 766–770.
Ma, G., Yang, D., Zhou, Y., Xiao, M., Kennedy, J. F., & Nie, J. (2008). Preparation and characterization of water-soluble N alkylated chitosan. Carbohydrate Polymer, 74, 121–126.
Majeti, N. V., & Kumar, R. (2000). A review of chitin and chitosan. Reactive and Functional Polymers, 46, 1–27.
Manjunatha, G., Roopa, K. S., Prashanth, G. N., & Shekar, S. H. (2008). Chitosan enhances disease resistance in pearl millet against downy mildew caused by Sclerospora graminicola and defence-related enzyme activation. Pest Management Sciences, 64, 1250–1257.
Menard, R., Alban, S., Ruffray, P., Jamois, F., Franz, G., Fritig, B., et al. (2004). β-1,3 Glucan sulfate, but not β-1,3 glucan, induces the salicylic acid signaling pathway in tobacco and Arabidopsis. The Plant Cell, 16, 3020–3032.
Meng, X. H., Li, B. Q., Liu, J., & Tian, S. P. (2008). Physiological responses and quality attributes of table grape fruit to chitosan preharvest spray and postharvest coating during storage. Food Chemistry, 106, 501–508.
Merkli, A., Christen, P., & Kapetanidis, I. (1997). Production of diosgenin by hairy root cultures of Trigonella foenum-graecum L. Plant Cell Reports, 16, 632–636.
Ning, W., Liu, Z. X., Li, Q., Guo, Z. J., & He, Z. H. (2003). Oligo saccharide oligo-GlcNAc induces hypersensitive cell death and enhances disease resistance in rice. Plant Physiology Communication, 39, 441–443.
No, H. K., Lee, K. S., Kim, I. D., Park, M. J., Kim, S. D., & Meyers, S. P. (2003). Chitosan treatment affects yield, ascorbic acid content, and hardness of soybean sprouts. Journal of Food Science, 68, 680–685.
Ortmann, I., & Moerschbacher, M. (2006). Spent growth medium of Pantoea agglomerans primes wheat suspension cells for augmented accumulation of hydrogen peroxide and enhanced peroxidase activity upon elicitation. Planta, 224, 963–970.
Park, S. I., & Zhao, Y. Y. (2004). Incorporation of a high concentration of mineral or vitamin into chitosan-based films. Journal Agricultural Food Chemistry, 52, 1933–1939.
Pospieszny, H. (1997). Antiviroid activity of chitosan. Crop Protection, 16, 105–106.
Qiuping, Z., & Wenshui, X. (2007). Effect of 1-methylcyclopropene and and/or chitosancoating treatments on storage life and quality maintenance of Indian jujube fruit. Lebensmittel-Wissenschaft und Technolnology, 40, 404–411.
Quang, L. L., Naotsugu, N., Masao, T., & Tomoko, N. (2006). Enhancement of plant growth activity of irradiated chitosan by molecular weight fractionation. Radioisotopes, 55, 23–27.
Raj, S. N., Sarosh, B. R., & Shetty, H. S. (2006). Induction and accumulation of polyphenol oxidase activities as implicated in development of resistance against pearl millet downy mildew disease. Functional Plant Biology, 33, 563–571.
Rakwal, R., Tamogami, S., Agrawal, G. K., & Iwahashi, H. (2002). Octadecanoid signaling component “burst” in rice (Oryza sativa L.) Seedling leaves upon wounding by cut and treatment with fungal elicitor chitosan. Biochemical and Biophysical Research Communications, 295, 1041–1045.
Schwabish, M. A., & Struhl, K. (2004). Evidence for eviction and rapid deposition of histones upon transcriptional elongation by RNA polymerase II. Molecular Cell Biology, 24, 10111–10117.
Sevon, N., Hiltunen, R., & Oksman-Caldentey, K. M. (1992). Chitosan increases hyoscyamine content in hairy root cultures of Hyoscyamus muticus. Pharmaceutical and Pharmacological Letters, 2, 96–99.
Shahidi, F., Arachchi, J. K. V., & Jeon, Y. J. (1999). Food applications of chitin and chitosans. Trends in Food Science and Technology, 10, 37–51.
Shao, C. X., Hu, J., Song, W. J., & Hu, W. M. (2005). Effects of seed priming with chitosan solutions of different acidity on seed germination and physiological characteristics of maize seedling. Agriculture and Life Science, 31, 705–708.
Sivakumar, D., Sultanbawa, Y., Ranasingh, N., Kumara, P., & Wijesundera, R. L. C. (2005). Effect of combined application of chitosan and carbonate salts on the incidence of anthracnose and on the quality of papaya during storage. Journal of Horticulture Sciences Biotechnology, 80, 447–452.
Sui, X. Y., Zhang, W. Q., Xia, W., & Wang, Q. (2002). Effect of chitosan as seed coating on seed germination and seedling growth and several physiological and biochemical indexes in rapeseed. Plant Physiology Communication, 38, 225–227.
Tan, T. K., Loon, W. S., Khor, E., & Loh, C. S. (1998). Infection of Spathoglottis plicata (Orchidaceae) seeds by mycorrhizal fungus. Plant Cell Reports, 18, 14–19.
Terry, L. A., & Joyce, D. C. (2004). Elicitors of induced disease resistance in postharvest horticultural crops: A brief review. Postharvest Biology and Technology, 32, 1–13.
Vander, P., Kjell, M. V., Domard, A., El-Gueddari, N. E., & Moerschbacher, B. M. (1998). Comparison of the ability of partially N-acetylated chitosans and oligosaccharides to elicit resistance in wheat leaves. Plant Physiology, 118, 1353–1359.
Vasyukova, N. I., Zinoveva, L. I., Iĺinskaya, E. A., Perekhod, G. I., Chalenko, N. G., Iĺina, A. V., et al. (2001). Modulation of plant resistance to diseases by water-soluble chitosan. Applied Biochemistry and Microbiology, 37, 103–109.
Weake, V. M., & Workman, J. I. (2008). Histone ubiquitination triggering gene activity. Molecular Cell, 29, 653–663.
White, P. J., & Broadley, M. (2003). Calcium in plants. Annals of Botany, 92, 487–511.
Wojdyla, A. T. (2004). Chitosan (biochikol 020 PC) in the control of some ornamental foliage diseases. Communications in Agricultural and Applied Biological Sciences, 69, 705–715.
Xu, Q. J., Nian, Y. G., Jin, X. C., Yan, C. Z., Liu, J., & Jiang, G. M. (2007). Effects of chitosan on growth of an aquatic plant (Hydrilla verticillata) in polluted waters with different chemical oxygen demands. Journal of Environmental Sciences, 19, 211–221.
Yamada, A., Shibbuya, N., Komada, O., & Akatsuka, T. (1993). Induction of phytoalexin formation in suspension-cultured rice cells by N-acetyl chitooligosaccharides. Biosciences Biotechnology Biochemistry, 57, 405–409.
Yin, H., Bai, X. F., & Du, Y. G. (2008). The primary study of oligochitosan inducing resistance to Sclerotinia scleraotiorum on B. napus. Journal of Biotechnology, 136, 600–601.
Yin, H., Zhao, X., & Du, Y. (2010). Oligochitosan a plant diseases vaccine—A review. Carbohydrate Polymer, 82, 1–8.
Yu, G., & Meuhlbauer, G. (2001). Benzothiadiazole-induced gene expression in wheat spikes does not provides resistance to Fusarium head blight. Physiological and Molecular Plant Pathology, 59, 129–139.
Yu, T., Wang, L. P., Yin, Y., Wang, Y. X., & Zheng, X. D. (2008). Effect of chitin on the antagonistic activity of Cryptococcus laurentii against Penicillium expansum in pear fruit. International Journal of Food Microbiology, 122, 44–48.
Zeng, D., & Luo, X. (2012). Physiological effects of chitosan coating on wheat growth and activities of protective enzyme with drought tolerance. Journal of Soil Science, 2, 282–288.
Zhang, X. K., Tang, Z. L., Zhan, L., et al. (2002). Influence of chitosan on induction rapeseed resistance. Agricultural Science in China, 35, 287–290.
Zhao, Y., Tu, K., Su, J., Tu, S., Hou, Y., et al. (2009). Heat treatment in combination with antagonistic yeast reduces diseases and elicits the active defense responses in harvested cherry tomato fruit. Journal Agriculture Food Chemistry, 57, 7565–7570.
Acknowledgments
We gratefully thanks the University Grant Commission New Delhi for financial support and special thanks to Department of Plant Physiology, Institute of Agricultural Sciences, Banaras Hindu University, Varanasi for providing necessary facilities for this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Katiyar, D., Hemantaranjan, A. & Singh, B. Chitosan as a promising natural compound to enhance potential physiological responses in plant: a review. Ind J Plant Physiol. 20, 1–9 (2015). https://doi.org/10.1007/s40502-015-0139-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40502-015-0139-6