Abstract
Cardiorenal syndrome type 1 (CRS-1) is the acute kidney disfunction caused by an acute worsening of cardiac function. CRS-1 is the consequence of renal vasoconstriction secondary to renin–angiotensin system (RAS) activation. No animal models of CRS-1 are described in literature.
Purpose
To characterize a murine model of CRS-1 by using a high-resolution ultrasound echo-color Doppler system (VEVO2100).
Materials
Post-ischemic heart failure was induced by coronary artery ligation (LAD) in seven CD1 mice. Fifteen and thirty days after surgery, mice underwent cardiac and renal echo-color Doppler. Serum creatinine and plasma renin activity were measured after killing. Animals were compared to seven CD1 control mice.
Results
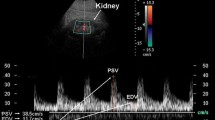
Heart failure with left ventricle dilatation (end diastolic area, p < 0.05 vs. controls) and significantly reduced ejection fraction (EF; p < 0.01 vs. controls) was evident 15 days after LAD. We measured a significant renal vasoconstriction in infarcted mice characterized by increased renal pulsatility index (PI; p < 0.05 vs. controls) associated to increased creatinine and renin levels (p < 0.05 vs. controls)
Conclusions
The mice model of LAD is a good model of CRS-1 evaluable by Doppler sonography and characterized by renal vasoconstriction due to the activation of the renin–angiotensin system secondary to heart failure.
RIASSUNTO
Per sindrome cardiorenale tipo-1 (SCR-1) si intende il peggioramento acuto della funzione renale dovuto alla riduzione della funzione cardiaca. La SCR-1 è conseguenza della vasocostrizione renale dovuta all’attivazione del sistema renina-angiotensina. In letteratura non sono descritti modelli animali che riproducono tale patologia.
Obiettivo di questo studio è caratterizzare mediante l’utilizzo di un sistema ecografico ad alta risoluzione (VEVO2100) un modello murino di CRS-1.
Materiali e Metodi
lo scompenso cardiaco post ischemico è stato indotto in 7 topi CD1 mediante legatura dell’arteria coronaria sinistra discendente anteriore. 15 e 30 giorni dopo la procedura chirurgica gli animali sono stati valutati mediante ecografia ed ecoDoppler cardiaco e renale. Gli animali sono stati sacrificati il trentesimo giorno e sono stati effettuati il dosaggio della creatinina sierica e dell’attività reninica plasmatica. I risultati sono stati confrontati con quelli ottenuti da 7 topi CD1 di controllo.
Risultati
la presenza di scompenso cardiaco, valutato mediante misurazione ecocardiografica dell’area telediastolica del ventricolo sinistro (EDA) e della frazione di eiezione (FE), è risultata evidente già 15 giorni dopo la LAD (rispettivamente p < 0.05 e p < 0.01 vs. controlli). E’ risultata inoltre una significativa vasocostrizione renale nei topi infartuati con aumento degli indici di pulsatilità renali (PI) nei topi infartuati (PI, p < 0.01 vs. controlli), associata ad un significativo aumento della creatinina e della attività reninica plasmatica (p < 0.05).
In conclusione
il modello murino di legatura di coronaria è un buon modello di SCR valutabile mediante ecoDoppler e caratterizzato da vasocostrizione renale e attivazione del sistema renina angiotensina.





Similar content being viewed by others
References
Bongartz LG, Cramer MJ, Braam B (2004) The cardiorenal connection. Hypertension 43:e14
Bongartz LG, Cramer MJ, Doevendans PA, Joles JA, Braam B (2005) The severe cardiorenal syndrome: ‘Guyton revisited’. Eur Heart J 26:11–17
Badzyńska B, Sadowski J (2011) Moderate intrarenal vasoconstriction after high pressor doses of norepinephrine in the rat: comparison with effects of angiotensin II. Kidney Blood Press Res 34:307–310
Sacerdoti D, Bolognesi M, Merkel C, Angeli P, Gatta A (1993) Renal vasoconstriction in cirrhosis evaluated by duplex Doppler ultrasonography. Hepatology 17:219–224
Monu SR, Pesce P, Sodhi K, Boldrin M, Puri N, Fedorova L, Sacerdoti D, Peterson SJ, Abraham NG (2013) Kappas A.HO-1 induction improves the type-1 cardiorenal syndrome in mice with impaired angiotensin II-induced lymphocyte activation. Hypertension 62(2):310–316
Mullens W, Abrahams Z, Francis GS et al (2009) Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol 53:589–596
Damman K, van Deursen VM, Navis G, Voors AA, van Veldhuisen DJ, Hillege HL (2009) Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol 53:582–588
Sinkeler SJ, Damman K, van Veldhuisen DJ, Hillege H, Navis G (2012) A re-appraisal of volume status and renal function impairment in chronic heart failure: combined effects of pre-renal failure and venous congestion on renal function. Heart Fail Rev 17(2):263–270
Zablocki D, Sadoshima J (2013) Angiotensin II and oxidative stress in the failing heart. Antioxid Redox Signal 19(10):1095–1109
Chu PY, Zatta A, Kiriazis H, Chin-Dusting J, Du XJ, Marshall T, Kaye DM (2011) CXCR4 antagonism attenuates the cardiorenal consequences of mineralocorticoid excess. Circ Heart Fail 4:651–658
Szymanski MK, de Boer RA, Navis GJ, van Gilst WH, Hillege HL (2012) Animal models of cardiorenal syndrome: a review. Heart Fail Rev 17:411–420
Lu J, Wang X, Wang W, Muniyappa H, Deshmukh A, Hu C, Das K, Mehta JL (2012) Abrogation of lectin-like oxidized LDL receptor-1 attenuates acute myocardial ischemia-induced renal dysfunction by modulating systemic and local inflammation. Kidney Int 82(4):436–444
VisualSonics VEVO2100 Operator Manual, Copyright © 2001–2008 by VisualSonics Inc
Benavides-Vallve C, Corbacho D, Iglesias-Garcia O, Pelacho B, Albiasu E, Castaño S, Muñoz Barrutia A, Prosper F, Ortiz-de-Solorzano C (2012) New strategies for echocardiographic evaluation of left ventricular function in a mouse model of long-term myocardial infarction. PLoS One 7(7):e41691
Francio SG, Gassler PJ Sonnenblick HE Fisiopatologia dello scompenso cardiaco. Il Cuore 10 ed. Hurst esd, McGraw-Hill Pubs 200; 743–79
Adiyanti SS, Loho T (2012) Acute kidney injury (AKI) biomarker. Acta Med Indones-Indones J Intern Med. 44(3):246–255
Verhave JC, Gansevoort RT, Hillege HL, De Zeeuw D, Curhan GC, De Jong PE (2004) Drawbacks of the use of indirect estimates of renal function to evaluate the effect of risk factors on renal function. J Am Soc Nephrol 15(5):1316–1322
Pfeffer MA, Pfeffer JM, Fishbein MC (1979) Myocardial infarct size and ventricular function in rats. Circ Res 44(4):503–512
Sjaastad I, Sejersted OM, Ilebekk A, Bjonerheim R (2000) Echocardiographic criteria for detection of postinfarction congestive heart failure in rats. J App Phisiol 89:1445–1454
Morgan E, Faulx M, McElfresh T, Kung T, Zawaneh M, Stanley W, Chandler M, Hoit B (2004) Validation of echocardiographic methods for assessing ventricular dysfunction in rats with myocardial infarction. Am J Phisiol Heart Circ Physiol 287(5):H2049–H2053
Fayssoil A Tournoux F (2012) Analyzing left ventricular function in mice with Doppler echocardiography. Heart Fail Rev
Sutton MG, Sharpe N (2000) Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation 101(2981–2988):41
Braunwald E (2001) Congestive heart failure: a half century perspective. Eur Heart J 22:825–836
Scherrer-Crosbie M, Thibault HB (2008) Echocardiography in translation research: of mice and men. J Am Soc Echocardiogr 21:1083–1092
Ganau A, Devereux RB, Roman MJ, de Simone G, Pickering TG, Saba PS, Vargiu P, Simongini I, Laragh JH (1992) Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J Am Coll Cardiol 19:1550–1558
Yuan Lijun, Wang Tao, Liu Fang, Cohen Ethan D, Patel Vickas V (2002) An evaluation of transmitral and pulmonary venous Doppler indices for assessing murine left ventricular diastolic function. Cardiol Rev. 10(4):218–229
ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 Appropriate use criteria for echocardiography a report of the American college of cardiology foundation appropriate use criteria task force, American society of echocardiography, American heart association, American Society of nuclear cardiology, heart failure society of America, heart rhythm society, society for cardiovascular angiography and interventions, society of critical care medicine, society of cardiovascular computed tomography, and society for cardiovascular magnetic resonance. Journal of the American College of Cardiology Vol. 57, No. 9, 2011 44
Lax JA, Bermann AM, Cianciulli TF, Morita LA, Masoli O, Prezioso HA (2000) Estimation of the ejection fraction in patients with myocardial infarction obtained from the combined index of systolic and diastolic left ventricular function: a new method. J Am Soc Echocardiogr 13(2):116
Lakoumentas JA, Panou FK, Kotseroglou VK, Aggeli KI, Harbis PK (2005) The Tei index of myocardial performance: applications in cardiology. Hellenic J Cardiol 46(1):52–58
Dujardin KS, Tei C, Yeo TC, Hodge DO, Rossi A, Seward JB (1998) Prognostic value of a Doppler index combining systolic and diastolic performance in idiopathic-dilated cardiomyopathy. Am J Cardiol 82(9):1071–1076
Ascione L, De Michele M, Accadia M, Rumolo S, Damiano L, D’Andrea A, Guarini P, Tuccillo B (2003) Myocardial global performance index as a predictor of in- hospital cardiac events in patients with first myocardial infarction. J Am Soc Echocardiogr 16(10):1019–1023
Conflict of interest
The authors declare that they have no conflict of interest.
Animal studies
The study was conducted in accordance with all institutional and national guidelines for the care and use of laboratory animals.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pesce, P., Cecchetto, L., Brocco, S. et al. Characterization of a murine model of cardiorenal syndrome type 1 by high-resolution Doppler sonography. J Ultrasound 18, 229–235 (2015). https://doi.org/10.1007/s40477-014-0129-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40477-014-0129-y