Abstract
Background
Osteoporosis and postmenopausal bone loss pose a huge social and economic burden worldwide. Regular exercise and physical activity are effective interventions for maximizing or maintaining peak bone mass and preventing bone loss in the elderly; however, most recommendations are addressed to the general public and lack specific indications for girls and women, the segment of the population most at risk for developing osteoporosis.
Objective
The aim of this overview of systematic reviews and meta-analyses was to summarize current evidence for the effects of exercise and physical activity interventions on bone status in girls and women, and to explore whether specific exercise programs exist for improving or maintaining bone mass or bone strength in females.
Methods
The PubMed, EMBASE, PEDro, and Cochrane Library databases were searched from January 2009, updated to 22 June 2015, using the following groups of search terms: (i) ‘physical activity’ and ‘exercise’; and (ii) ‘bone’, ‘bone health’, ‘bone strength’, ‘bone structure’, ‘bone metabolism’, ‘bone turnover’, and ‘bone biomarkers’. Searches and screening were limited to systematic reviews or meta-analyses of studies in females and published in English. Our final analysis included 12 articles that met the inclusion criteria.
Results
Combined-impact exercise protocols (impact exercise with resistance training) are the best choice to preserve/improve bone mineral density in pre- and postmenopausal women. Peak bone mass in young girls can be improved with short bouts of school-based high-impact plyometric exercise programs. Whole-body vibration exercises have no beneficial effects on bone in postmenopausal or elderly women.
Conclusions and Implications
Lifelong exercise, specific for age, is an effective way to sustain bone health in girls and women.
Similar content being viewed by others
Age-specific, lifelong exercise is an effective way to sustain bone status in females. |
Combined-impact exercise protocols are the best choice to preserve/improve bone mineral density in pre- and postmenopausal women, while short bouts of school-based high-impact plyometric exercise programs can effectively improve peak bone mass in young girls. |
1 Introduction
According to a World Health Organization (WHO) report, physical inactivity has become a global public health problem worldwide, prevalently affecting women and accounting for approximately 3.2 million deaths each year [1]. There is abundant evidence documenting that regular physical activity and exercise can improve overall health and fitness and prevent many adverse health outcomes. As such, they constitute an important component of a healthy lifestyle in both men and women [2, 3] (physical activity and exercise as defined elsewhere [3, 4]). Moderate to strong empirical evidence has shown that among the countless and well-demonstrated benefits regular physical activity and exercise can bring as non-pharmacological interventions, it can improve bone health in children or adolescents, adults, and the elderly [2, 5].
Bone health is essential for overall health and quality of life. In addition to providing the body with a frame for mobility and protection against injury, bone tissue is a storehouse of minerals vital for the normal functioning of other organs, including hematopoiesis and physiological regulation of endocrine organs [6–9]. Unhealthy bones lead to dysfunction and fractures, resulting in disability, diminished function, loss of independence, and even premature death [6]. Osteoporosis, the most common bone disease in humans, has become a worldwide health problem. It is characterized by low bone mass, deterioration of bone tissue, and disruption of bone architecture with compromised bone strength and increased risk of fractures. One of every two Caucasian women will suffer an osteoporosis-related fracture over her lifetime, as will approximately one in five men. The cost of healthcare for an aging population is estimated to reach $25.3 billion in 2025 in the US alone [10].
Due to the increasing prevalence of osteoporosis (and osteoporosis-related diseases) and its staggering cost in postmenopausal women, research has focused on the effects of exercise as an effective non-pharmacological intervention for maximizing peak bone mass in girls (during growth), maintaining or minimizing bone loss in middle-aged and premenopausal women, and reducing bone loss rate and preventing falls and fractures in postmenopausal and older women. Areal bone mineral density (aBMD) and bone mineral content (BMC), as measured by dual x-ray absorptiometry (DXA), or volumetric BMD (vBMD) and bone strength indexes (BSIs), as measured by peripheral quantitative computed tomography (pQCT), are commonly used to study bone health and predict fracture risk [10–14]. In addition to static measures by radiological and densitometric techniques, bone biomarkers can be used as complementary dynamic measures to obtain serial information about the instantaneous metabolic activity of bone cells and of bone health at the molecular level [8].
Organizations such as the WHO, the American College of Sports Medicine (ACSM), the National Institutes of Health (NIH), and the National Osteoporosis Foundation (NOF) have issued exercise recommendations to promote bone health and to prevent and treat osteoporosis [5, 10, 15–17]. However, mMost of the recommendations are general and are not specifically addressed to either males or females and/or to one age group. More interestingly, studies about the benefits of exercise on bone status and osteoporosis prevention are focused on women. Studies in men are still lacking, although it might be considered that the prevalence of osteopenia and osteoporosis in males is dramatically growing due to increasing ageing [16]. Recently, systematic reviews and meta-analyses on the effects of exercise programs on bone in girls, premenopausal women, and postmenopausal or older women, along with randomized control trials (RCTs) or prospective cohorts, have begun to provide new insights. Since high-quality systematic reviews and meta-analyses can be recognized as level 1 evidence for public health interventions, an overview of high-quality systematic reviews and meta-analyses can summarize robust evidence from exercise programs for bone health in different age-stage female subjects [18, 19].
To our knowledge, there is no overview of systematic reviews and meta-analyses on the effects of exercise on bone status in female subjects from childhood to older age. Therefore, we performed the present overview in order to assemble current evidence on this topic. Moreover, we wanted to determine whether specific exercise intervention programs exist for improving bone status from the early through to later years of life.
2 Methods
This overview of systematic reviews and meta-analyses was conducted according to prespecified eligibility criteria, search strategies, data sources, study selection, data extraction, and subgroup analyses. Suggestions for the methodology in conducting a systematic review of systematic reviews of healthcare interventions were taken from Smith et al. [20].
2.1 Inclusion Criteria
This overview only included systematic reviews or meta-analyses on the effects of exercise on bone parameters of healthy female subjects, from girls to postmenopausal or older women, without pathological diseases affecting bone metabolism, as measured by DXA or pQCT and supported (or not) by measurement of biomarkers of bone metabolism. Systematic reviews or meta-analyses on subjects with primary osteoporosis induced by ageing rather than secondary diseases were also included. Systematic reviews or meta-analyses in athletes or mixed samples of athletes and general female subjects without separate subgroup analyses were excluded. Only articles published in English were included.
If several systematic reviews or meta-analyses fulfilled the inclusion criteria, we included only one review per combination of age-stage, exercise intervention, and outcome by choosing the latest review with a high-quality score or the highest number of studies included [19].
2.2 Data Sources and Search Strategies
The PubMed, EMBASE, PEDro, and Cochrane Library databases were searched from January 2009 to December 2014. The choice of 2009 as a starting point derived from the fact that in that year the latest PRISMA (Preferred Reporting Items for Systematic Reviews) guidelines for conducting high-quality systematic reviews or meta-analyses were published and promoted in academic circles [21, 22]. We expected to retrieve high-quality systematic reviews following the PRISMA guidelines. Two groups of terms were matched and used in the literature search as follows: (i) ‘physical activity’ and ‘exercise’; and (ii) ‘bone’, ‘bone health’, ‘bone strength’, ‘bone structure’, ‘bone metabolism’, ‘bone turnover’, and ‘bone biomarkers’. Filters were activated in all four databases for systematic reviews or meta-analyses from 2009 to the search date. Reference lists of the included articles were also hand-searched. Moreover, during manuscript preparation, a second updated literature search was conducted to 22 June 2015 in order to include the most recent studies. Data searches were performed by the first author (JX) and monitored by the second author (GL).
2.3 Study Selection and Data Extraction
All abstracts and titles were read and then screened by the first author for potentially relevant studies based on the inclusion criteria. Full-text articles of selected titles or abstracts were retrieved and screened for eligibility by the first author. The second author then reviewed the study selection and screening, and disagreements were solved by consensus.
Data were extracted following the PICO rule, i.e. characteristics of participants, interventions, comparisons, and outcome measures. Most systematic reviews or meta-analyses included comparisons of exercise programs addressed to improving a variety of bone parameters versus control subjects who either did not perform the exercises or performed sham exercises. Since all outcome measures of bone status, as measured by DXA or pQCT in the systematic reviews or meta-analyses, were continuous, the weighted mean difference (WMD) or the standardized mean difference (SMD) with a 95 % confidence interval (CI) was summarized if possible. Subgroup pooled effects of exercise protocols in different age-group female subjects were also extracted where applicable, as reported in the systematic reviews or meta-analyses.
2.4 Quality Assessment of Systematic Reviews or Meta-Analyses
AMSTAR, a recently developed and widely accepted tool, was used to assess systematic reviews [23, 24]. This tool is composed of 11 items, each with a ‘yes’, ‘no’, ‘can’t answer’, or ‘not applicable’ option; the item rated as ‘yes’ can be awarded 1 point (Table 1). Generally, AMSTAR scores of 0–4 indicate low quality, 5–8 indicate moderate quality, and 9–11 indicate high quality [25, 26]. Quality assessment was performed by the first author and reviewed by the second author. Disagreements were solved by consensus.
3 Results
3.1 Article Selection, Characteristics, and Quality
3.1.1 Systematic Reviews or Meta-Analyses Search and Selection
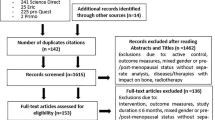
In all, 973 references were identified in the four databases (Fig. 1). After title and abstract screening, 927 references were excluded. Of the remaining 46 references, 12 duplicate items were excluded, leaving 34 references for full-text retrieval and screening. Eighteen articles were excluded and the remaining 16 articles were evaluated for quality and final selection and screening. All reference lists of these 16 articles were hand-searched and one additional study was retrieved. Six articles were excluded because of low methodological quality or for other reasons. Furthermore, one article was included in the second literature update search and screening, based on the same search and inclusion criteria. Finally, 12 articles [11–14, 27–34] were included for final overview and analysis.
3.1.2 Characteristics of the Systematic Reviews or Meta-Analyses
Only one [14] of the 12 systematic reviews or meta-analyses was a Cochrane systematic review, and the remaining 11 articles [11–13, 27–34] were non-Cochrane systematic reviews. Eight articles [11–14, 28, 30, 32, 33] focused on female subjects, including girls and premenopausal or postmenopausal women. Four articles [27, 29, 31, 34] analyzed mixed cohorts of males and females, with subgroup systematic reviews or meta-analyses of female subjects. Three articles [11, 27, 34] analyzed exercise effects on bone mass and strength in girls (children or adolescents), five articles [12, 13, 27, 33, 34] summarized the effects of exercise on young female adults or premenopausal women, and seven articles [14, 28–32, 34] included postmenopausal or older women. Six articles [14, 28–31, 34] included only RCTs, five articles [11–13, 32, 33] included controlled trials (both RCTs and non-RCTs), and one article [27] included only cohort studies. All articles used parameters such as aBMD or vBMD, BMC, BSI, or stress-strain index (SSI), as measured by DXA or pQCT. Without special explanations, BMD denotes aBMD in the tables or contexts of the present overview. In the absence of specific information indicating otherwise, BMD denotes aBMD in this overview. No bone biomarkers were used or analyzed in any of the reviews. Eight articles [14, 27–31, 33, 34] mentioned funding sources for the studies, one article [11] reported no support had been received, and three articles [12, 13, 32] did not report funding status. Table 2 presents the general characteristics of the reviews.
3.1.3 Methodological Quality Assessment
One article was rated as being of low quality (AMSTAR score of 4) [11], eight articles [12, 27–31, 33, 34] were rated as being of moderate quality (score 5–8), and three articles [13, 14, 32] were rated as being of high quality (scores 9–10). The low-quality article, as assessed by AMSTAR, was included because it was the only comprehensive systematic review with a quantitative meta-analysis that focused exclusively on girls. The majority of the systematic reviews or meta-analyses (11 articles) were of moderate to high quality. AMSTAR quality assessment scores are shown in Table 2, with items rated ‘yes’ in the AMSTAR checklist.
3.2 Effects of Exercise
The effects of exercises on bone were classified by age group: young girls (children or adolescents), young adult women (premenopause), and postmenopausal or older women.
3.2.1 Young Girls (Children or Adolescents)
3.2.1.1 Overall Effects
Ishikawa et al. [11] reported that weight-bearing exercises significantly increase BMC of the femoral neck (WMD 0.23, 95 % CI 0.10–0.36) and lumbar spine (WMD 0.19, 95 % CI 0.05–0.33), and lumbar spine BMD (WMD 0.26, 95 % CI 0.09–0.43) in healthy young girls without previous experience in organized physical training programs. Bielemann et al. [27] found that, despite the lack of association between physical activity during adolescence and bone mass in young adulthood, there was a positive association between cumulative physical activity from adolescence to adulthood and bone mass in young adulthood. In the review by Nikander et al. [34], no significant effect of exercise on bone strength-related parameters (BSI, SSI, section modulus [Z], or cross-sectional moment of inertia [CSMI]) in young girls was observed. The overall effects of exercises are presented in Table 3.
3.2.1.2 Sensitivity or Subgroup Analyses in Reviews
Only the review by Ishikawa et al. included sensitivity or subgroup analyses according to different exercise program settings or maturity stages of young girls [11]. A significant overall effect of physical exercise on total-body BMD (TB-BMD) was only found in early pubertal girls (Tanner stage II–III). Significant overall effects were only detected in femoral neck BMC in subgroup analyses of both early pubertal and pubertal girls (Tanner stage IV–V), plyometric exercise programs, school-based exercise programs, exercise programs lasting <60 min/week, exercise programs for ≤3 days/week, and exercise programs lasting <12 months and ≥12 months. Subgroup analyses found significant overall effects in lumbar spine BMC and BMD. BMC was improved in early pubertal girls and BMD was improved in pre-pubertal girls. Plyometric exercise programs, school-based programs, exercise programs lasting <60 min/week or ≥60 min/week, exercise programs for ≤3 days/week or >3 days/week, and exercise programs lasting ≥12 months increased both BMC and BMD significantly. Significant sensitivity or subgroup analyses of exercises are shown in Table 4.
3.2.1.3 Exercise Programs
In the review by Ishikawa et al. [11], exercise programs were classified as either plyometric (jumping, hopping, skipping, bounding, etc.) or non-plyometric weight-bearing exercises (weight training, muscle strength exercises, etc.) lasting from 26 weeks to 24 months. Exercise frequency varied from 1 to 5 days/week in school-based or non-school-based settings. Exercise duration was from 10 to 20 min for most plyometric exercises, to 30–45 min for non-plyometric weight-bearing exercises. Conversely, in the review by Nikander et al. [34], exercise programs were described as short bouts of school-based high-impact interventions lasting 10–15 min. Program length varied from 7 to 24 months and frequency varied from two to five times per week. Bielemann et al. [27] did not mention specific exercise programs, but only reviewed the relationship between physical activity level in young girls and BMC or BMD in cohort studies. No data regarding compliance with the exercise interventions were reported in these three systematic reviews. The exercise programs are detailed in Table 3.
3.2.2 Young Adult Women (Premenopause)
3.2.2.1 Overall Effects
In the meta-analysis by Zhao et al. [33], femoral neck BMD (WMD 0.017, 95 % CI 0.014–0.20) and trochanter BMD (WMD 0.021, 95 % CI 0.018–0.024) were significantly improved by high-impact and short-duration jumping exercise. Bielemann et al. [27] found no association between general physical activity during adulthood and bone mass measurements. In the review by Babatunde et al. [12], femoral neck BMD (SMD 0.53, 95 % CI 0.26–0.79) and trochanter BMD (SMD 0.22, 95 % CI −0.04 to 0.49) were found to be significantly increased after short bouts of high-impact exercises, while Nikander et al. [34] found no significant overall effects of exercise on bone-strength-related parameters. Martyn-St James and Carroll [13] found lumbar spine BMD (WMD 0.006, 95 % CI 0.002–0.010) and femoral neck BMD (WMD 0.012, 95 % CI 0.001–0.022) were improved after different impact exercise programs (high impact, odd impact, and combination). The overall effects are presented in Table 5.
3.2.2.2 Sensitivity or Subgroup Analyses in Reviews
Sensitivity or subgroup analyses were only performed in the review by Martyn-St James and Carroll [13]. Results showed that combined (impact and resistance exercises) protocols, all combined studies lasting ≥10 months, and all combined studies with attrition rates ≤30 % could effectively improve lumbar spine BMD in premenopausal women. Significant improvements were noted in femoral neck BMD after both high-impact exercises alone and combined exercise protocols. All combined studies lasting ≥10 months were also effective in improving femoral neck BMD. Significant sensitivity or subgroup analyses are shown in Table 6.
3.2.2.3 Exercise Programs
Zhao et al. [33] specifically examined high-impact and short-duration two-legged jumping exercises. Generally, the jumping exercise protocols were home- or office-based and consisted of several bouts of two-legged jumps with short rest intervals (8–15 s); the programs lasted from 6 to 12 months for three to seven times per week. Compliance with the exercise programs was not reported, although authors provided information about reasons for dropout [33]. Bielemann et al. [27] did not mention specific exercise programs. In the article by Babatunde et al. [12], short bouts of home- or office-based high-impact exercise was reviewed, and was described as one to five bouts of 10–20 jumps with rest times between bouts. Protocol length was from 4 to 12 months, and frequency was from 3 to 7 days/week. Compliance with the exercise interventions ranged from 69 to 86 %. Nikander et al. [34] reported on 60-min workout classes for 12 months that included stepping and hopping three times per week plus 10 min of brief steps and hops daily. Martyn-St James and Carroll [13] reviewed different types of impact exercises classified as high impact (vertical jumping, skipping, etc.), odd impact (bench stepping, hurdle bounding, etc.), and combined loading exercises (odd impact or high impact exercises with resistance training or weighted-vest work). Protocol length was between 6 and 24 months, with a duration varying from several bouts of jumps to 60-min workouts for 1–3 days/week. Compliance with exercise ranged between 45 and 91 %. Exercise programs are detailed in Table 5.
3.2.3 Postmenopausal or Older Women
3.2.3.1 Overall Effects
Polidoulis et al. [28] reported that exercises could significantly improve trabecular volumetric BMD (Tv-BMD; WMD 0.87, 95 % CI 0.37–1.37) of the distal tibia, and cortical volumetric BMD (Cv-BMD; WMD 0.89, 95 % CI 0.37–1.41) of the tibial shaft, as measured by pQCT in healthy postmenopausal women. Marques et al. [29] found that exercises significantly increased lumbar spine BMD (WMD 0.012, 95 % CI 0.002–0.022) and femoral neck BMD (WMD 0.014, 95 % CI 0.003–0.025) in older women. Kelley et al. [30] found that different reaction force exercises could effectively improve femoral neck BMD (SMD 0.208, 95 % CI 0.102–0.474) and lumbar spine BMD (SMD 0.179, 95 % CI −0.003 to 0.361) in postmenopausal women who were not regularly active, as defined by the authors. Lau et al. [31] found no significant overall effects of whole-body vibration exercises (WBV) in older women, and the Cochrane review by Howe et al. [14] reported that exercise improved lumbar spine (SMD 0.85, 95 % CI 0.62–1.07) and trochanter BMD (SMD 1.03, 95 % CI 0.56–1.49) in healthy postmenopausal women. Nikander et al. [34] found no effective improvements in bone-strength-related parameters after exercise in postmenopausal or older women, while Martyn-St James and Carroll [32] demonstrated that impact exercises may improve lumbar spine BMD (WMD 0.015, 95 % CI 0.005–0.025), femoral neck BMD (WMD 0.008, 95 % CI 0.004–0.013), and total hip BMD (WMD 0.013, 95 % CI 0.001–0.024) in sedentary postmenopausal women. The overall effects are shown in Table 7.
3.2.3.2 Sensitivity or Subgroup Analyses in Reviews
Sensitivity or subgroup analyses were performed in three reviews [14, 29, 32]. Marques et al. [29] found that odd-impact exercises and exercise programs for 3 days/week or more could often improve both lumbar spine BMD and femoral neck BMD, while combined loading exercises (resistance/strength exercises with endurance and/or balance exercises) improved only lumbar spine BMD. Howe et al. [14] found that hip BMD can be improved with static weight-bearing exercises (SWB, including single-leg standing) and dynamic weight-bearing exercises with high force (DWBHF, including jogging, jumping, running, dancing, and vibration platform); lumbar spine BMD can be improved with dynamic weight-bearing exercises with low force (DWBLF, including walking and Tai chi), non-weight-bearing exercises with high force (NWBHF, e.g. progressive resistance training) and combination exercises (COMB, more than one of the above exercise interventions); trochanter BMD can be improved with DWBHF and COMB exercises; and femoral neck BMD can be improved with NWBHF and COMB exercises. Martyn-St James and Carroll [32] found that both combined-impact exercises (odd-impact/high-impact classes combined with resistance training) and low-impact exercises (jogging combined with walking/stair climbing) can improve lumbar spine BMD and femoral neck BMD. Significant sensitivity or subgroup analyses are shown in Table 8.
3.2.3.3 Exercise Programs
In the review by Polidoulis et al. [28], exercise protocols were generally classified as low-impact, high-impact, resistance, and agility exercises. Program length was from 5 to 12 months, and frequency was from two to five times per week. Exercise duration was between 45 and 60 min, and compliance with the exercise protocols ranged from 50 to 85 %. In the review by Marques et al. [29], most exercise programs were center-based and included either resistance training exercises or a combination of a resistance/strength component with endurance and/or balance exercises. Program length was from 6 to 30 months, and frequency from 2 to 4 days/week. Exercise duration was between approximately 15 and 90 min, and compliance ranged from 67 to 99 %. Kelley et al. [30] classified exercises as joint reaction force (strength/resistance training), ground reaction force (aerobic exercises, including walking, jumping, etc.), and combination exercises. Program length was between 6 months and 2 years, and frequency ranged from 2 to 6 days/week. Exercise duration was mainly from 10 to 90 min, and compliance with the exercise program ranged from 39 to 95 %. Lau et al. [31] focused on WBV exercises exclusively. Programs of WBV intervention lasted from 24 weeks to 18 months, with one to seven sessions per week. A session could include from 1 to 27 bouts lasting from 30 s to 30 min, with rest intervals between bouts. Supervision of WBV was either indicated or not indicated. The Cochrane review by Howe et al. [14] classified six different types of exercises: SWB, DWBLF, DWBHF, NWBLF (non-weight-bearing exercise, low force), NWBHF, and COMB. Twelve months were used as the cutoff point for the description of program lengths of the studies included. Exercise frequency in the majority of the studies was two to three times per week, and the exercise duration ranged from 30 to 60 min. Compliance with the exercise programs ranged from 39 to 95 %. High-impact circuit training, resistance training, balance jumping, agility training, multidirectional jumps and steps, and combination exercises were included in the review by Nikander et al. [34]. Program length was between 6 and 12 months, and frequency was from two to six times per week. Exercise duration of 20 min was reported in only one study included in this review. Martyn-St James and Carroll [32] classified exercise programs into four types: high-impact loading exercises (vertical jumping, rope jumping, fast running, etc.), odd-impact loading exercises (aerobic or step classes, bounding, agility exercises, etc.), low-impact loading exercises (slow jogging etc.), and combined loading exercises (impact exercises mixed with strength/resistance training). Program length lasted from 5 to 26 months, and frequency was from 2 to 7 days/week. Exercise programs were indicated as being either supervised or unsupervised, and compliance with the exercise interventions ranged from 50 to 91 %. The exercise programs are detailed in Table 7.
4 Discussion
4.1 Effects of Exercise on Bone in Girls
Based on the overall evidence and results of the systematic reviews or meta-analyses in young, healthy girls (pre- or pubertal girls) (Table 3), we may summarize that weight-bearing exercises (brief plyometrics or non-plyometric weight-bearing exercises) can effectively improve BMC and/or BMD in specific sites, such as the femoral neck and lumbar spine [11]. Regular physical activity starting from adolescence through to adulthood positively correlated with BMD or BMC in young adult women [27]; however, it seems that short bouts of impact exercises (plyometric) do not significantly influence bone-strength-related parameters, as measured by pQCT, such as distal tibial BSI, tibial midshaft SSI, femoral neck Z and CSMI [34]. Based on sexual maturity grouping (Table 4) [11], short bouts of high-impact exercise programs (plyometrics; duration 10–20 min/time, ≥60 min/week; frequency ≥3 days/week; length ≥12 months; setting school-based) should be recommended for obtaining exercise-related improvements in bone mass and quality (particularly with respect to BMD) in young (pre- or pubertal) girls. Early puberty (Tanner stages II and III) seems to be the age-stage most sensitive to the effects of exercise on BMC and BMD in young growing girls [11]. These findings are generally in line with the official recommendations of the ACSM or US Department of Health and Human Services (DHHS) on physical activity or exercise and bone health in children or adolescents [6, 16].
In animal studies, intermittent dynamic impact loading cycles have been shown to improve bone-strength parameters (ultimate force [F u] and energy to failure [U]) with relatively small gains in BMC and BMD [35]. However, in the systematic reviews included in the present analysis, dynamic impact loading exercises (plyometrics) were reported to significantly improve BMD in young healthy girls (although minimally) without any direct positive changes in bone strength parameters, as measured by pQCT. The explanation for this phenomenon could be the different bone strength parameters used in animal and human studies, respectively, or the BMD changes induced by different interventions or different biological mechanisms. It is reasonable to be cautious when applying animal research results to human beings, and further research is desirable to clarify the reasons for these discrepancies. Furthermore, we should also note that BMD at best accounts for 60–70 % of variance in ultimate bone strength, since bone material properties, bone cellular activities, bone microarchitecture, and bone geometry are also recognized as determinants of bone strength [28, 36].
In summary, besides following general recommendations by official health and sports organizations [6, 16], we should note that short bouts of school-based, high-impact plyometric exercise programs (duration 10–20 min/time (≥60 min/week); frequency ≥3 days/week; overall length ≥12 months) may maximize benefits in improving BMD in young girls, especially early pubertal girls (Tanner stages II and III) [11]. To this end, education administration departments could make a big difference in promoting school-based exercise programs to enhance bone health in young girls.
4.2 Effects of Exercise on Bone in Premenopausal Women
Based on the evidence and results of the systematic reviews or meta-analyses in premenopausal women (Table 5), we may conclude that general physical activity does not further improve bone mass [27]; specific impact exercises, such as high impact (plyometric jumping/hopping) and combined impact (odd- or high-impact exercise with resistance training) can significantly improve BMD in specific sites of the femoral neck, lumbar spine, and trochanter [12, 13, 33]. As found in young girls, exercise did not improve bone-strength parameters, as measured by pQCT. Further subgroup or sensitivity analyses showed that combined-impact exercise protocols can increase BMD in both the femoral neck and the lumbar spine, but short duration and high-impact exercise protocols improved only femoral neck BMD or trochanter BMD (Table 6) [12, 13, 33]. A longer, combined-impact protocol (≥10 months) might lead to more gains in lumbar spine and femoral neck BMD. These findings are also in line with general recommendations by the ACSM for adults [16] and a specific part of the DHHS guidelines for young adults [6]. However, we should note that general physical activity did not further improve bone mass in premenopausal women. Only specific high-impact or combined-impact exercise protocols may be effective.
In summary, in addition to following the general recommendations by official health and sports organizations [6, 16] to maximize the effects of exercise in maintaining or minimizing bone loss in premenopausal women, where tolerable or applicable, combined-impact exercise protocols (odd-impact or high-impact exercise with resistance training; duration 30–60 min; frequency ≥3 days/week; overall length ≥10 months) may be recommended for better bone health benefits. For women who lack time because of family or work commitments, short bouts of home- or office-based intermittent high-impact exercises (vertical jumps or hops; 1–5 bouts, 10–20 vertical jumps/bout, 8–15 s interval rest between jumps or bouts; ≥3 days/week; length ≥6 months) could be the better choice [12, 33].
4.3 Effects of Exercise on Bone in Postmenopausal and Older Women
Owing to the higher prevalence of osteoporosis in postmenopausal or older women, numerous clinical studies and systematic reviews or meta-analyses have investigated the effects of exercise on bone in this population segment. The only Cochrane systematic review included in the present overview focused on this population and had the highest methodological quality score of 10 [14]. More importantly, exercise is the only single intervention that can simultaneously improve muscle mass and strength, balance, and bone health [6]. Based on the overall evidence and results of the systematic reviews or meta-analyses in postmenopausal or older women (Table 7), long-term weight-bearing exercises or resistance training or combined-impact exercises (weight-bearing impact with resistance training) (generally lasting ≥6 months; frequency ≥3 days/week) may significantly preserve bone mass or avert bone loss in specific sites compared with control groups [14, 28–30, 32]; however, the recently developed WBV exercise modality was found to have no statistically significant effect in preserving bone mass compared with control groups [31]. As found in young girls and premenopausal women, exercise did not lead to changes in bone-strength parameters, as measured by pQCT [28, 34]. On the contrary, bone-structure parameters, Tv-BMD and Cv-BMD, as measured by pQCT, gained significant improvements in tibia bone [28]. This result differed from those obtained in animals, which may be explained by the bone-strength parameters used or the degree of BMD change induced by different interventions or different biological mechanisms. The general findings of the articles are in line with official ACSM or DHHS recommendations [5, 6, 16].
Moreover, similar findings have been shown in further subgroup or sensitivity analyses (Table 8). In all three systematic reviews or meta-analyses that conducted subgroup or sensitivity analyses [14, 29, 32], combined-impact exercises (impact exercises, especially odd impact/high impact, with resistance training) were effective in improving the BMD of specific sites in postmenopausal women; however, some discrepancies were also noted. For example, the sensitivity analyses by Marques et al. [29] showed that odd-impact exercises can effectively improve BMD in the lumbar spine and femoral neck, while Martyn-St James and Carroll [32] found that low-impact exercises (jogging combined with walking/stair climbing/cycling) improved BMD in the lumbar spine and femoral neck more so than odd-impact exercises. Possible reasons for this difference is that the literature search deadlines (February 2011 vs. March 2008) included subjects (only older women vs. postmenopausal women) and other studies (RCTs vs. controlled trials). The results combined with the highest-quality Cochrane review conclusions [14] show that a combined-impact exercise protocol is the most effective for improving BMD in postmenopausal or older women.
In summary, in addition to following general recommendations [5, 6, 16], combined-impact exercise protocols (impact, especially odd-impact/high-impact exercises with resistance training; duration 30–60 min; frequency ≥3 days/week; overall length ≥10 months) represent the best choice to preserve/improve BMD in postmenopausal women, where tolerable and applicable. The new concept of WBV seems to have no additional effects in preserving bone mass compared with control groups.
4.4 Limitations and Implications
For the reasons explained above, we set the literature search starting date at January 2009. The majority of the articles were of moderate quality, as assessed using AMSTAR [23]: one article had a low-quality score [11], and only three articles had high-quality scores [13, 14, 32]. To date, no high-quality systematic review or meta-analysis of studies in young growing girls has been conducted. Furthermore, high-quality systematic reviews or meta-analyses following recognized guidelines will need to be carried out to confirm our overall findings, especially in young girls. None of the articles were awarded points for item 11 in the AMSTAR evaluation, which requires not only reporting the source of funding or support for the systematic review but also for each of the studies included therein. Future systematic reviews or meta-analyses should pay attention to this requirement.
To some degree, BMD changes, as measured by radiological methods, are the static results of exercise effects on bone, whereas bone metabolism biomarkers can more sensitively reflect acute and dynamic metabolic changes in bone [8, 37]. To our surprise, none of the articles included in the systematic reviews or meta-analyses reported the use of bone biomarkers. Future studies should include bone biomarker measurements in the study design to complement radiological measurements in order to better understand the effects of exercise on bone.
Compliance with the exercise programs varied dramatically from 39 to 99 % in systematic reviews included in the present overview. Future studies should analyze the underlying reasons for low compliance in certain settings in order to improve exercise promotion in females. Lastly, as mentioned above, no difference was observed between males and females with respect to exercise programs in official recommendations. Current studies regarding the benefits of exercise on bone status and osteoporosis prevention are focused on women, while such studies in men are still lacking [16]. Hence, investigating the effects on men of the exercise programs summarized in the current review will be worthwhile.
5 Conclusions
Lifelong physical activity or regular exercise is critically important for improving bone health in girls and women. In young girls, early puberty seems to be the most sensitive stage for maximizing bone mass gains. Short bouts of school-based high-impact exercise protocols could be the best choice for improving bone mass in young growing girls. In young premenopausal women, combined-impact exercise protocols have the most comprehensive effects on BMD; however, short bouts of home- or office-based impact exercise protocols could also make a difference. In postmenopausal women, where tolerable and applicable, combined-impact exercise protocols should be initially recommended for preserving bone mass or improving BMD. These exercise programs could also be useful in men in terms of bone health promotion and osteoporosis prevention. Official recommendations or guidelines from recognized health and sports organizations should also be followed.
Most importantly, the age-specific exercise programs for females presented in the current review are based on the results of the most recent systematic reviews, which means healthcare personnel can promote or prescribe them for bone-health promotion or osteoporosis prevention in the knowledge that the exercises involved can be performed safely and correctly. As described in the position stand of ACSM [16], “Maintaining a vigorous level of physical activity across the lifespan should be viewed as an essential component of the prescription for achieving and maintaining good bone health”.
References
World Health Organization. Physical inactivity: a global public health problem. 2014. Available at: http://www.who.int/dietphysicalactivity/factsheet_inactivity/en/. Accessed July 2014.
US Department of Health and Human Services. 2008 physical activity guidelines for Americans. Washington, DC: US Department of Health and Human Services; 2008.
Pate RR, Pratt M, Blair SN, et al. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA. 1995;273(5):402–7.
Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100(2):126–31.
Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, et al. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41(7):1510–30.
US Department of Health and Human Services. Bone health and osteoporosis: a report of the Surgeon General. Rockville (MD): US Department of Health and Human Services, Office of the Surgeon General; 2004.
Karsenty G, Ferron M. The contribution of bone to whole-organism physiology. Nature. 2012;481(7381):314–20.
Banfi G, Lombardi G, Colombini A, et al. Bone metabolism markers in sports medicine. Sports Med. 2010;40(8):697–714.
Lombardi G, Perego S, Luzi L, et al. A four-season molecule: osteocalcin. Updates in its physiological roles. Endocrine. 2015;48(2):394–404.
National Osteoporosis Foundation. Clinician’s gudie to prevention and treatment of osteoporosis. Washington, DC: National Osteoporosis Foundation; 2014.
Ishikawa S, Kim Y, Kang M, et al. Effects of weight-bearing exercise on bone health in girls: a meta-analysis. Sports Med. 2013;43(9):875–92.
Babatunde OO, Forsyth JJ, Gidlow CJ. A meta-analysis of brief high-impact exercises for enhancing bone health in premenopausal women. Osteoporos Int. 2012;23(1):109–19.
Martyn-St James M, Carroll S. Effects of different impact exercise modalities on bone mineral density in premenopausal women: a meta-analysis. J Bone Miner Metab. 2010;28(3):251–67.
Howe TE, Shea B, Dawson LJ, et al. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev. 2011;(7):CD000333.
World Health Organization. Global recommendations on physical activity for health. Geneva: World Health Organization; 2010.
Kohrt WM, Bloomfield SA, Little KD, et al. American College of Sports Medicine Position Stand: physical activity and bone health. Med Sci Sports Exerc. 2004;36(11):1985–96.
National Institute of Health. Osteoporosis prevention, diagnosis, and therapy. NIH Consens Statement. 2000;17(1):1–36.
OCEBM Levels of Evidence Working Group. The Oxford 2011 Levels of Evidence. Oxford Centre for Evidence-Based Medicine. 2011. Available at: http://www.cebm.net/index.aspx?o=5653. Accessed May 2014.
Hagen KB, Dagfinrud H, Moe RH, et al. Exercise therapy for bone and muscle health: an overview of systematic reviews. BMC Med. 2012;10:167.
Smith V, Devane D, Begley CM, et al. Methodology in conducting a systematic review of systematic reviews of healthcare interventions. BMC Med Res Methodol. 2011;11(1):15.
Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100.
Shea BJ, Hamel C, Wells GA, et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol. 2009;62(10):1013–20.
Shea BJ, Grimshaw JM, Wells GA, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7:10.
Seo HJ, Kim KU. Quality assessment of systematic reviews or meta-analyses of nursing interventions conducted by Korean reviewers. BMC Med Res Methodol. 2012;12:129.
Mikton C, Butchart A. Child maltreatment prevention: a systematic review of reviews. Bull World Health Organ. 2009;87(5):353–61.
Bielemann RM, Martinez-Mesa J, Gigante DP. Physical activity during life course and bone mass: a systematic review of methods and findings from cohort studies with young adults. BMC Musculoskelet Disord. 2013;14:77.
Polidoulis I, Beyene J, Cheung AM. The effect of exercise on pQCT parameters of bone structure and strength in postmenopausal women: a systematic review and meta-analysis of randomized controlled trials. Osteoporos Int. 2012;23(1):39–51.
Marques EA, Mota J, Carvalho J. Exercise effects on bone mineral density in older adults: a meta-analysis of randomized controlled trials. Age. 2012;34(6):1493–515.
Kelley GA, Kelley KS, Kohrt WM. Effects of ground and joint reaction force exercise on lumbar spine and femoral neck bone mineral density in postmenopausal women: a meta-analysis of randomized controlled trials. BMC Musculoskelet Disord. 2012;13:177.
Lau RW, Liao LR, Yu F, et al. The effects of whole body vibration therapy on bone mineral density and leg muscle strength in older adults: a systematic review and meta-analysis. Clin Rehabil. 2011;25(11):975–88.
Martyn-St James M, Carroll S. A meta-analysis of impact exercise on postmenopausal bone loss: the case for mixed loading exercise programmes. Br J Sports Med. 2009;43(12):898–908.
Zhao R, Zhao M, Zhang L. Efficiency of jumping exercise in improving bone mineral density among premenopausal women: a meta-analysis. Sports Med. 2014;44(10):1393–402.
Nikander R, Sievanen H, Heinonen A, et al. Targeted exercise against osteoporosis: a systematic review and meta-analysis for optimising bone strength throughout life. BMC Med. 2010;8:47.
Turner CH, Robling AG. Designing exercise regimens to increase bone strength. Exerc Sport Sci Rev. 2003;31(1):45–50.
Fonseca H, Moreira-Goncalves D, Coriolano HJ, et al. Bone quality: the determinants of bone strength and fragility. Sports Med. 2014;44(1):37–53.
Maimoun L, Sultan C. Effects of physical activity on bone remodeling. Metabolism. 2011;60(3):373–88.
Acknowledgments
The authors are indebted to Mr. Kenneth Britsch for language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported independently by the China Scholarship Council (Grant No. 201306520008) and the Italian Ministry of Health.
Conflict of interest
Jincheng Xu, Giovanni Lombardi, Wei Jiao, and Giuseppe Banfi declare that they have no conflicts of interest relevant to the content of this review.
Rights and permissions
About this article
Cite this article
Xu, J., Lombardi, G., Jiao, W. et al. Effects of Exercise on Bone Status in Female Subjects, from Young Girls to Postmenopausal Women: An Overview of Systematic Reviews and Meta-Analyses. Sports Med 46, 1165–1182 (2016). https://doi.org/10.1007/s40279-016-0494-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-016-0494-0