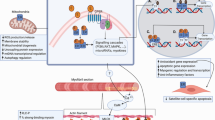
Abstract
Skeletal muscle regeneration efficiency declines with age for both men and women. This decline impacts on functional capabilities in the elderly and limits their ability to engage in regular physical activity and to maintain independence. Aging is associated with a decline in sex hormone production. Therefore, elucidating the effects of sex hormone substitution on skeletal muscle homeostasis and regeneration after injury or disuse is highly relevant for the aging population, where sarcopenia affects more than 30 % of individuals over 60 years of age. While the anabolic effects of androgens are well known, the effects of estrogens on skeletal muscle anabolism have only been uncovered in recent times. Hence, the purpose of this review is to provide a mechanistic insight into the regulation of skeletal muscle regenerative processes by both androgens and estrogens. Animal studies using estrogen receptor (ER) antagonists and receptor subtype selective agonists have revealed that estrogens act through both genomic and non-genomic pathways to reduce leukocyte invasion and increase satellite cell numbers in regenerating skeletal muscle tissue. Although animal studies have been more conclusive than human studies in establishing a role for sex hormones in the attenuation of muscle damage, data from a number of recent well controlled human studies is presented to support the notion that hormonal therapies and exercise induce added positive effects on functional measures and lean tissue mass. Based on the fact that aging human skeletal muscle retains the ability to adapt to exercise with enhanced satellite cell activation, combining sex hormone therapies with exercise may induce additive effects on satellite cell accretion. There is evidence to suggest that there is a ‘window of opportunity’ after the onset of a hypogonadal state such as menopause, to initiate a hormonal therapy in order to achieve maximal benefits for skeletal muscle health. Novel receptor subtype selective ligands and selective estrogen and androgen receptor modulators (SERMs, SARMs) promise to reduce health risks associated with classical hormonal therapies, whilst maintaining the positive effects on muscle repair. Dietary supplements containing compounds with structural similarity to estrogens (phytoestrogens) are increasingly used as alternatives to classical hormone-replacement therapies (HRT), but the effects on skeletal muscle are currently largely unknown. Research has started to investigate the combined effects of exercise and alternative HRTs, such as soy isoflavones, on skeletal muscle regenerative processes to provide safer and more efficient therapies to promote muscle regeneration and maintenance of muscle mass and strength in the aging population.
Similar content being viewed by others
References
Dreyer HC, Blanco CE, Sattler FR, et al. Satellite cell numbers in young and older men 24 hours after eccentric exercise. Muscle Nerve. 2006;33(2):242–53. doi:10.1002/mus.20461.
Carlson BM, Faulkner JA. Muscle transplantation between young and old rats: age of host determines recovery. Am J Physiol. 1989;256(6 Pt 1):C1262-6.
Velders M, Schleipen B, Fritzemeier KH, et al. Selective estrogen receptor-beta activation stimulates skeletal muscle growth and regeneration. FASEB J. 2012;26(5):1909–20. doi:10.1096/fj.11-194779.
Enns DL, Iqbal S, Tiidus PM. Oestrogen receptors mediate oestrogen-induced increases in post-exercise rat skeletal muscle satellite cells. Acta Physiol (Oxf). 2008;194(1):81–93.
Enns DL, Tiidus PM. Estrogen influences satellite cell activation and proliferation following downhill running in rats. J Appl Physiol. 2008;104(2):347–53.
Ferry A, Noirez P, Page CL, et al. Effects of anabolic/androgenic steroids on regenerating skeletal muscles in the rat. Acta Physiol Scand. 1999;166(2):105–10.
Bhasin S, Woodhouse L, Storer TW. Proof of the effect of testosterone on skeletal muscle. J Endocrinol. 2001;170(1):27–38. pii: JOE04157.
Roth SM, Martel GF, Ivey FM, et al. Skeletal muscle satellite cell characteristics in young and older men and women after heavy resistance strength training. J Gerontol Ser A Biol Sci Med Sci. 2001;56(6):B240–7.
Carlson BM, Gutmann E. Development of contractile properties of minced muscle regenerates in the rat. Exp Neurol. 1972;36(2):239–49. pii: 0014-4886(72)90020-9.
Turner NJ, Badylak SF. Regeneration of skeletal muscle. Cell Tissue Res. 2012;347(3):759–74. doi:10.1007/s00441-011-1185-7.
Belcastro AN, Shewchuk LD, Raj DA. Exercise-induced muscle injury: a calpain hypothesis. Mol Cell Biochem. 1998;179(1–2):135–45.
Wehling M, Spencer MJ, Tidball JG. A nitric oxide synthase transgene ameliorates muscular dystrophy in mdx mice. J Cell Biol. 2001;155(1):123–31. doi:10.1083/jcb.200105110155/1/123.
Tidball JG, Villalta SA. Regulatory interactions between muscle and the immune system during muscle regeneration. Am J Physiol Regul Integr Comp Physiol. 2010;298(5):R1173–87.
Tidball JG. Inflammatory processes in muscle injury and repair. Am J Physiol Regul Integr Comp Physiol. 2005;288(2):R345–53.
Saclier M, Yacoub-Youssef H, Mackey AL, et al. Differentially activated macrophages orchestrate myogenic precursor cell fate during human skeletal muscle regeneration. Stem Cells. 2012. doi:10.1002/stem.1288.
Shireman PK, Contreras-Shannon V, Ochoa O, et al. MCP-1 deficiency causes altered inflammation with impaired skeletal muscle regeneration. J Leukoc Biol. 2007;81(3):775–85.
Bencze M, Negroni E, Vallese D, et al. Proinflammatory macrophages enhance the regenerative capacity of human myoblasts by modifying their kinetics of proliferation and differentiation. Mol Ther. 2012;20(11):2168–79. doi:10.1038/mt.2012.189.
Tedesco FS, Dellavalle A, Diaz-Manera J, et al. Repairing skeletal muscle: regenerative potential of skeletal muscle stem cells. J Clin Invest. 2010;120(1):11–9. doi:10.1172/JCI40373.
Mikkelsen UR, Langberg H, Helmark IC, et al. Local NSAID infusion inhibits satellite cell proliferation in human skeletal muscle after eccentric exercise. J Appl Physiol. 2009;107(5):1600–11.
Mackey AL, Kjaer M, Dandanell S, et al. The influence of anti-inflammatory medication on exercise-induced myogenic precursor cell responses in humans. J Appl Physiol. 2007;103(2):425–31. doi:10.1152/japplphysiol.00157.2007.
Ferrari G, Cusella-De Angelis G, Coletta M, et al. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279(5356):1528–30.
Dellavalle A, Maroli G, Covarello D, et al. Pericytes resident in postnatal skeletal muscle differentiate into muscle fibres and generate satellite cells. Nat Commun. 2011;2:499. doi:10.1038/ncomms1508.
Sambasivan R, Yao R, Kissenpfennig A, et al. Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development. 2011;138(17):3647–56. doi:10.1242/dev.067587.
Lepper C, Partridge TA, Fan CM. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development. 2011;138(17):3639–46. doi:10.1242/dev.067595.
Kuang S, Charge SB, Seale P, et al. Distinct roles for Pax7 and Pax3 in adult regenerative myogenesis. J Cell Biol. 2006;172(1):103–13.
Collins CA, Olsen I, Zammit PS, et al. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122(2):289–301. doi:10.1016/j.cell.2005.05.010.
Cheung TH, Quach NL, Charville GW, et al. Maintenance of muscle stem-cell quiescence by microRNA-489. Nature. 2012;482(7386):524–8. doi:10.1038/nature10834.
Zammit PS, Heslop L, Hudon V, et al. Kinetics of myoblast proliferation show that resident satellite cells are competent to fully regenerate skeletal muscle fibers. Exp Cell Res. 2002;281(1):39–49. pii: S0014482702956533.
Hawke TJ, Garry DJ. Myogenic satellite cells: physiology to molecular biology. J Appl Physiol. 2001;91(2):534–51.
Wierman ME. Sex steroid effects at target tissues: mechanisms of action. Adv Physiol Educ. 2007;31(1):26–33. doi:10.1152/advan.00086.2006.
Beato M, Klug J. Steroid hormone receptors: an update. Hum Reprod Update. 2000;6(3):225–36.
Wierman ME, Kohrt WM. Vascular and metabolic effects of sex steroids: new insights into clinical trials. Reprod Sci. 2007;14(4):300–14. doi:10.1177/1933719107303673.
Wyce A, Bai Y, Nagpal S, et al. Research resource: the androgen receptor modulates expression of genes with critical roles in muscle development and function. Mol Endocrinol. 2010;24(8):1665–74. doi:10.1210/me.2010-0138.
Lindberg MK, Moverare S, Skrtic S, et al. Estrogen receptor (ER)-beta reduces ERalpha-regulated gene transcription, supporting a “ying yang” relationship between ERalpha and ERbeta in mice. Mol Endocrinol. 2003;17(2):203–8.
Kumar MV, Leo ME, Tindall DJ. Modulation of androgen receptor transcriptional activity by the estrogen receptor. J Androl. 1994;15(6):534–42.
Milanesi L, Russo de Boland A, Boland R. Expression and localization of estrogen receptor alpha in the C2C12 murine skeletal muscle cell line. J Cell Biochem. 2008;104(4):1254–73.
Milanesi L, Vasconsuelo A, de Boland AR, et al. Expression and subcellular distribution of native estrogen receptor beta in murine C2C12 cells and skeletal muscle tissue. Steroids. 2009;74(6):489–97.
Lemoine S, Granier P, Tiffoche C, et al. Effect of endurance training on oestrogen receptor alpha transcripts in rat skeletal muscle. Acta Physiol Scand. 2002;174(3):283–9.
Lemoine S, Granier P, Tiffoche C, et al. Effect of endurance training on oestrogen receptor alpha expression in different rat skeletal muscle type. Acta Physiol Scand. 2002;175(3):211–7.
Wiik A, Glenmark B, Ekman M, et al. Oestrogen receptor beta is expressed in adult human skeletal muscle both at the mRNA and protein level. Acta Physiol Scand. 2003;179(4):381–7.
Wiik A, Ekman M, Morgan G, et al. Oestrogen receptor beta is present in both muscle fibres and endothelial cells within human skeletal muscle tissue. Histochem Cell Biol. 2005;124(2):161–5.
Wiik A, Ekman M, Johansson O, et al. Expression of both oestrogen receptor alpha and beta in human skeletal muscle tissue. Histochem Cell Biol. 2009;131(2):181–9.
Hall JM, Couse JF, Korach KS. The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem. 2001;276(40):36869–72. doi:10.1074/jbc.R100029200R100029200.
Dubois V, Laurent M, Boonen S, et al. Androgens and skeletal muscle: cellular and molecular action mechanisms underlying the anabolic actions. Cell Mol Life Sci. 2012;69(10):1651–67. doi:10.1007/s00018-011-0883-3.
Christian HC, Rolls NJ, Morris JF. Nongenomic actions of testosterone on a subset of lactotrophs in the male rat pituitary. Endocrinology. 2000;141(9):3111–9.
Karas RH, Hodgin JB, Kwoun M, et al. Estrogen inhibits the vascular injury response in estrogen receptor beta-deficient female mice. Proc Natl Acad Sci USA. 1999;96(26):15133–6.
McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr Rev. 1999;20(3):279–307.
Pietras RJ, Szego CM. Specific binding sites for oestrogen at the outer surfaces of isolated endometrial cells. Nature. 1977;265(5589):69–72.
Chambliss KL, Yuhanna IS, Anderson RG, et al. ERbeta has nongenomic action in caveolae. Mol Endocrinol. 2002;16(5):938–46.
Simoncini T, Genazzani AR. Non-genomic actions of sex steroid hormones. Eur J Endocrinol. 2003;148(3):281–92.
Fu R, Liu J, Fan J, et al. Novel evidence that testosterone promotes cell proliferation and differentiation via G protein-coupled receptors in the rat L6 skeletal muscle myoblast cell line. J Cell Physiol. 2012;227(1):98–107. doi:10.1002/jcp.22710.
Estrada M, Espinosa A, Muller M, et al. Testosterone stimulates intracellular calcium release and mitogen-activated protein kinases via a G protein-coupled receptor in skeletal muscle cells. Endocrinology. 2003;144(8):3586–97.
Rahman F, Christian HC. Non-classical actions of testosterone: an update. Trends Endocrinol Metab. 2007;18(10):371–8. doi:10.1016/j.tem.2007.09.004.
Hamdi MM, Mutungi G. Dihydrotestosterone activates the MAPK pathway and modulates maximum isometric force through the EGF receptor in isolated intact mouse skeletal muscle fibres. J Physiol. 2010;588(Pt 3):511–25. doi:10.1113/jphysiol.2009.182162.
Bär PR, Amelink GJ, Oldenburg B, et al. Prevention of exercise-induced muscle membrane damage by oestradiol. Life Sci. 1988;42(26):2677–81.
Amelink GJ, Bär PR. Exercise-induced muscle protein leakage in the rat: effects of hormonal manipulation. J Neurol Sci. 1986;76(1):61–8.
Bär PR, Amelink GJ. Protection against muscle damage exerted by oestrogen: hormonal or antioxidant action? Biochem Soc Trans. 1997;25(1):50–4.
Friden J, Lieber RL. Serum creatine kinase level is a poor predictor of muscle function after injury. Scand J Med Sci Sports. 2001;11(2):126–7.
Amelink GJ, Koot RW, Erich WB, et al. Sex-linked variation in creatine kinase release, and its dependence on oestradiol, can be demonstrated in an in-vitro rat skeletal muscle preparation. Acta Physiol Scand. 1990;138(2):115–24.
Persky AM, Green PS, Stubley L, et al. Protective effect of estrogens against oxidative damage to heart and skeletal muscle in vivo and in vitro. Proc Soc Exp Biol Med. 2000;223(1):59–66.
Tiidus PM, Holden D, Bombardier E, et al. Estrogen effect on post-exercise skeletal muscle neutrophil infiltration and calpain activity. Can J Physiol Pharmacol. 2001;79(5):400–6.
Tiidus PM. Can oestrogen influence skeletal muscle damage, inflammation, and repair? Br J Sports Med. 2005;39(5):251–3.
Stupka N, Tiidus PM. Effects of ovariectomy and estrogen on ischemia–reperfusion injury in hindlimbs of female rats. J Appl Physiol. 2001;91(4):1828–35.
McClung JM, Davis JM, Carson JA. Ovarian hormone status and skeletal muscle inflammation during recovery from disuse in rats. Exp Physiol. 2007;92(1):219–32.
McClung JM, Davis JM, Wilson MA, et al. Estrogen status and skeletal muscle recovery from disuse atrophy. J Appl Physiol. 2006;100(6):2012–23.
McLennan IS. Resident macrophages (ED2- and ED3-positive) do not phagocytose degenerating rat skeletal muscle fibres. Cell Tissue Res. 1993;272(1):193–6.
Massimino ML, Rapizzi E, Cantini M, et al. ED2+ macrophages increase selectively myoblast proliferation in muscle cultures. Biochem Biophys Res Commun. 1997;235(3):754–9.
Enns DL, Tiidus PM. The influence of estrogen on skeletal muscle: sex matters. Sports Med. 2010;40(1):41–58.
Whiting KP, Restall CJ, Brain PF. Steroid hormone-induced effects on membrane fluidity and their potential roles in non-genomic mechanisms. Life Sci. 2000;67(7):743–57.
Ghisletti S, Meda C, Maggi A, et al. 17beta-estradiol inhibits inflammatory gene expression by controlling NF-kappaB intracellular localization. Mol Cell Biol. 2005;25(8):2957–68.
Pierce AP, de Waal E, McManus LM, et al. Oxidation and structural perturbation of redox-sensitive enzymes in injured skeletal muscle. Free Radic Biol Med. 2007;43(12):1584–93.
Barton ER, Morris L, Musaro A, et al. Muscle-specific expression of insulin-like growth factor I counters muscle decline in mdx mice. J Cell Biol. 2002;157(1):137–48.
Tiidus PM, Deller M, Liu XL. Oestrogen influence on myogenic satellite cells following downhill running in male rats: a preliminary study. Acta Physiol Scand. 2005;184(1):67–72.
Thomas A, Bunyan K, Tiidus PM. Oestrogen receptor-alpha activation augments post-exercise myoblast proliferation. Acta Physiol (Oxf). 2010;198(1):81–9.
Koller M. Weight changes in the anterior tibial muscle of the prepuberal rat in normal conditions, after castration and after treatment with 2 anabolic steroids. Boll Soc Ital Biol Sper. 1960;36:756–9.
Kuc A, Poplawski W, Targonski R, et al. Influence of nerobolil on regeneration and histochemical reactions in striated muscles. Folia Morphol (Warsz). 1973;32(1):89–101.
Serra C, Tangherlini F, Rudy S, et al. Testosterone improves the regeneration of old and young mouse skeletal muscle. J Gerontol Ser A Biol Sci Med Sci. 2013;68(1):17–26. doi:10.1093/gerona/gls083.
Wagner Alves de Souza R, Goncalves W, Garrido Cavalcante WL, et al. Nandrolone stimulates MyoD expression during muscle regeneration in the condition of myonecrosis induced by Bothrops jararacussu venom poisoning. J Toxicol Environ Health A. 2010;73(13–14):934–43. doi:10.1080/15287391003751729.
Tamaki T, Uchiyama Y, Okada Y, et al. Anabolic-androgenic steroid does not enhance compensatory muscle hypertrophy but significantly diminish muscle damages in the rat surgical ablation model. Histochem Cell Biol. 2009;132(1):71–81. doi:10.1007/s00418-009-0584-2.
Chen Y, Zajac JD, MacLean HE. Androgen regulation of satellite cell function. J Endocrinol. 2005;186(1):21–31.
Thompson RW, McClung JM, Baltgalvis KA, et al. Modulation of overload-induced inflammation by aging and anabolic steroid administration. Exp Gerontol. 2006;41(11):1136–48. doi:10.1016/j.exger.2006.08.013.
Tiidus PM, Enns DL. Point:counterpoint: estrogen and sex do/do not influence post-exercise indexes of muscle damage, inflammation, and repair. J Appl Physiol. 2009;106(3):1010–2 (discussion 4–15, 21). doi:10.1152/japplphysiol.90848.2008.
Pizza FX, Clark BC, De Meersman RE, et al. Comments on Point:counterpoint: estrogen and sex do/do not influence post-exercise indexes of muscle damage, inflammation, and repair. J Appl Physiol. 2009;106(3):1016–20. doi:10.1152/japplphysiol.00004.2009.
Ronkainen PH, Kovanen V, Alen M, et al. Postmenopausal hormone replacement therapy modifies skeletal muscle composition and function: a study with monozygotic twin pairs. J Appl Physiol. 2009;107(1):25–33. doi:10.1152/japplphysiol.91518.2008.
Taaffe DR, Sipila S, Cheng S, et al. The effect of hormone replacement therapy and/or exercise on skeletal muscle attenuation in postmenopausal women: a yearlong intervention. Clin Physiol Funct Imaging. 2005;25(5):297–304. doi:10.1111/j.1475-097X.2005.00628.x.
MacNeil LG, Baker SK, Stevic I, et al. 17beta-estradiol attenuates exercise-induced neutrophil infiltration in men. Am J Physiol Regul Integr Comp Physiol. 2011;300(6):R1443–51. doi:10.1152/ajpregu.00689.2009.
Dieli-Conwright CM, Spektor TM, Rice JC, et al. Hormone therapy and maximal eccentric exercise alters myostatin-related gene expression in postmenopausal women. J Strength Condition Res. 2012;26(5):1374–82. doi:10.1519/JSC.0b013e318251083f.
Dieli-Conwright CM, Spektor TM, Rice JC, et al. Influence of hormone replacement therapy on eccentric exercise induced myogenic gene expression in postmenopausal women. J Appl Physiol. 2009;107(5):1381–8. doi:10.1152/japplphysiol.00590.2009.
Dieli-Conwright CM, Spektor TM, Rice JC, et al. Hormone therapy attenuates exercise-induced skeletal muscle damage in postmenopausal women. J Appl Physiol. 2009;107(3):853–8.
Dieli-Conwright CM, Spektor TM, Rice JC, et al. HRT and mRNA expression of estrogen receptor coregulators following exercise in postmenopausal women. Med Sci Sports Exerc. 2010;42:422–9.
Dieli-Conwright CM, Spektor TM, Rice JC, et al. Oestradiol and SERM treatments influence oestrogen receptor coregulator gene expression in human skeletal muscle cells. Acta Physiol (Oxf). 2009;197(3):187–96. doi:10.1111/j.1748-1716.2009.01997.x.
Sipila S, Taaffe DR, Cheng S, et al. Effects of hormone replacement therapy and high-impact physical exercise on skeletal muscle in post-menopausal women: a randomized placebo-controlled study. Clin Sci (Lond). 2001;101(2):147–57.
Choi SD, Steinberg EM, Lee HH, et al. The timing hypothesis remains a valid explanation of differential cardioprotective effects of menopausal hormone treatment. Menopause. 2011;18(2):230–6.
Schierbeck LL, Rejnmark L, Tofteng CL, et al. Effect of hormone replacement therapy on cardiovascular events in recently postmenopausal women: randomised trial. BMJ. 2012;345:e6409. doi:10.1136/bmj.e6409.
Vingren JL, Kraemer WJ, Ratamess NA, et al. Testosterone physiology in resistance exercise and training: the up-stream regulatory elements. Sports Med. 2010;40(12):1037–53. doi:10.2165/11536910-000000000-00000.
Bhasin S, Storer TW, Berman N, et al. Testosterone replacement increases fat-free mass and muscle size in hypogonadal men. J Clin Endocrinol Metab. 1997;82(2):407–13.
Doumit ME, Cook DR, Merkel RA. Testosterone up-regulates androgen receptors and decreases differentiation of porcine myogenic satellite cells in vitro. Endocrinology. 1996;137(4):1385–94.
Bhasin S, Storer TW, Berman N, et al. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med. 1996;335(1):1–7.
Bhasin S, Storer TW, Javanbakht M, et al. Testosterone replacement and resistance exercise in HIV-infected men with weight loss and low testosterone levels. JAMA. 2000;283(6):763–70.
Lee DK. Androgen receptor enhances myogenin expression and accelerates differentiation. Biochem Biophys Res Commun. 2002;294(2):408–13.
Sinha-Hikim I, Roth SM, Lee M, et al. Testosterone-induced muscle hypertrophy is associated with an increase in satellite cell number in healthy, young men. Am J Physiol Endocrinol Metab. 2003;285(1):e197–205. doi:10.1152/ajpendo.00370.200200370.2002.
Zhao W, Pan J, Wang X, et al. Expression of the muscle atrophy factor muscle atrophy F-box is suppressed by testosterone. Endocrinology. 2008;149(11):5449–60. doi:10.1210/en.2008-0664.
Liva SM, Voskuhl RR. Testosterone acts directly on CD4+ T lymphocytes to increase IL-10 production. J Immunol. 2001;167(4):2060–7.
Cappola AR, Ratcliffe SJ, Bhasin S, et al. Determinants of serum total and free testosterone levels in women over the age of 65 years. J Clin Endocrinol Metab. 2007;92(2):509–16. doi:10.1210/jc.2006-1399.
Sheffield-Moore M, Paddon-Jones D, Casperson SL, et al. Androgen therapy induces muscle protein anabolism in older women. J Clin Endocrinol Metab. 2006;91(10):3844–9. doi:10.1210/jc.2006-0588.
Kraemer WJ, Hakkinen K, Newton RU, et al. Effects of heavy-resistance training on hormonal response patterns in younger vs. older men. J Appl Physiol. 1999;87(3):982–92.
Liao YH, Liao KF, Kao CL, et al. Effect of dehydroepiandrosterone administration on recovery from mix-type exercise training-induced muscle damage. Eur J Appl Physiol. 2013;113(1):99–107. doi:10.1007/s00421-012-2409-6.
Craft RM, Clark JL, Hart SP, et al. Sex differences in locomotor effects of morphine in the rat. Pharmacol Biochem Behav. 2006;85(4):850–8. doi:10.1016/j.pbb.2006.11.022.
Bronstein PM, Wolkoff FD, Levine WJ. Sex-related differences in rats open-field activity. Behav Biol. 1975;13(1):133–8.
Hertrampf T, Degen GH, Kaid AA, et al. Combined effects of physical activity, dietary isoflavones and 17beta-estradiol on movement drive, body weight and bone mineral density in ovariectomized female rats. Planta Med. 2006;72(6):484–7. doi:10.1055/s-2006-931579.
Bowen RS, Turner MJ, Lightfoot JT. Sex hormone effects on physical activity levels: why doesn’t Jane run as much as Dick? Sports Med. 2011;41(1):73–86. doi:10.2165/11536860-000000000-00000.
Greising SM, Baltgalvis KA, Lowe D, et al. Hormone therapy and skeletal muscle strength: a meta-analysis. J Gerontol Ser A Biol Sci Med Sci. 2009;64:1071–81.
Maltais ML, Desroches J, Dionne IJ. Changes in muscle mass and strength after menopause. J Musculoskelet Neuronal Interact. 2009;9(4):186–97.
Phillips SK, Rook KM, Siddle NC, et al. Muscle weakness in women occurs at an earlier age than in men, but strength is preserved by hormone replacement therapy. Clin Sci (Lond). 1993;84(1):95–8.
Diel P, Friedel A, Geyer H, et al. Characterisation of the pharmacological profile of desoxymethyltestosterone (Madol), a steroid misused for doping. Toxicol Lett. 2007;169(1):64–71.
Gao W, Reiser PJ, Coss CC, et al. Selective androgen receptor modulator treatment improves muscle strength and body composition and prevents bone loss in orchidectomized rats. Endocrinology. 2005;146(11):4887–97. doi:10.1210/en.2005-0572.
Jones A, Hwang DJ, Narayanan R, et al. Effects of a novel selective androgen receptor modulator on dexamethasone-induced and hypogonadism-induced muscle atrophy. Endocrinology. 2010;151(8):3706–19. doi:10.1210/en.2010-0150.
Dalton JT, Barnette KG, Bohl CE, et al. The selective androgen receptor modulator GTx-024 (enobosarm) improves lean body mass and physical function in healthy elderly men and postmenopausal women: results of a double-blind, placebo-controlled phase II trial. J Cachexia Sarcopenia Muscle. 2011;2(3):153–61. doi:10.1007/s13539-011-0034-6.
Clarke BL, Khosla S. New selective estrogen and androgen receptor modulators. Curr Opin Rheumatol. 2009;21(4):374–9. doi:10.1097/BOR.0b013e32832ca447.
Runowicz CD, Costantino JP, Wickerham DL, et al. Gynecologic conditions in participants in the NSABP breast cancer prevention study of tamoxifen and raloxifene (STAR). Am J Obstet Gynecol. 2011;205(6):535 e1–5. doi:10.1016/j.ajog.2011.06.067.
Koot RW, Amelink GJ, Blankenstein MA, et al. Tamoxifen and oestrogen both protect the rat muscle against physiological damage. J Steroid Biochem Mol Biol. 1991;40(4–6):689–95.
Grata E, Perrenoud L, Saugy M, et al. SARM-S4 and metabolites detection in sports drug testing: a case report. Forensic Sci Int. 2011;213(1–3):104–8. doi:10.1016/j.forsciint.2011.07.014.
Hayashi SI, Eguchi H, Tanimoto K, et al. The expression and function of estrogen receptor alpha and beta in human breast cancer and its clinical application. Endocr Relat Cancer. 2003;10(2):193–202.
Harris HA. The unexpected science of estrogen receptor-beta selective agonists: a new class of anti-inflammatory agents? Nucl Recept Signal. 2006;4:e012.
Velders M, Solzbacher M, Schleipen B, et al. Estradiol and genistein antagonize the ovariectomy effects on skeletal muscle myosin heavy chain expression via ER-beta mediated pathways. J Steroid Biochem Mol Biol. 2010;120(1):53–9. doi:10.1016/j.jsbmb.2010.03.059.
Weigt C, Hertrampf T, Zoth N, et al. Impact of estradiol, ER subtype specific agonists and genistein on energy homeostasis in a rat model of nutrition induced obesity. Mol Cell Endocrinol. 2012;351(2):227–38. doi:10.1016/j.mce.2011.12.013.
Schleipen B, Hertrampf T, Fritzemeier KH, et al. ERbeta-specific agonists and genistein inhibit proliferation and induce apoptosis in the large and small intestine. Carcinogenesis. 2011;32(11):1675–83. doi:10.1093/carcin/bgr188.
Hertrampf T, Schleipen B, Velders M, et al. Estrogen receptor subtype-specific effects on markers of bone homeostasis. Mol Cell Endocrinol. 2008;291(1–2):104–8. doi:10.1016/j.mce.2008.03.003.
Cvoro A, Tatomer D, Tee MK, et al. Selective estrogen receptor-beta agonists repress transcription of proinflammatory genes. J Immunol. 2008;180(1):630–6.
Harris HA, Albert LM, Leathurby Y, et al. Evaluation of an estrogen receptor-beta agonist in animal models of human disease. Endocrinology. 2003;144(10):4241–9.
Minutolo F, Macchia M, Katzenellenbogen BS, et al. Estrogen receptor beta ligands: recent advances and biomedical applications. Med Res Rev. 2011;31:364–442.
Kuiper GG, Carlsson B, Grandien K, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138(3):863–70.
Pihlajamaa P, Zhang FP, Saarinen L, et al. The phytoestrogen genistein is a tissue-specific androgen receptor modulator. Endocrinology. 2011;152(11):4395–405. doi:10.1210/en.2011-0221.
Kalbe C, Mau M, Rehfeldt C. Developmental changes and the impact of isoflavones on mRNA expression of IGF-I receptor, EGF receptor and related growth factors in porcine skeletal muscle cell cultures. Growth Horm IGF Res. 2008;18(5):424–33.
Jones KL, Harty J, Roeder MJ, et al. In vitro effects of soy phytoestrogens on rat L6 skeletal muscle cells. J Med Food. 2005;8(3):327–31.
Rehfeldt C, Kalbe C, Nurnberg G, et al. Dose-dependent effects of genistein and daidzein on protein metabolism in porcine myotube cultures. J Agric Food Chem. 2009;57(3):852–7.
Serra MC, Beavers KM, Beavers DP, et al. Effects of 28 days of dairy or soy ingestion on skeletal markers of inflammation and proteolysis in post-menopausal women. Nutr Health. 2012;21(2):117–30. doi:10.1177/0260106012467243.
Hubal MJ, Clarkson PM. Counterpoint: estrogen and sex do not significantly influence post-exercise indexes of muscle damage, inflammation, and repair. J Appl Physiol. 2009;106(3):1012–4 (discussion 4, 22) doi:10.1152/japplphysiol.90848.2008a.
Balagopal P, Olney R, Darmaun D, et al. Oxandrolone enhances skeletal muscle myosin synthesis and alters global gene expression profile in Duchenne muscular dystrophy. Am J Physiol Endocrinol Metab. 2006;290(3):e530–9. doi:10.1152/ajpendo.00412.2005.
Onambele-Pearson GL. HRT affects skeletal muscle contractile characteristics: a definitive answer? J Appl Physiol. 2009;107(1):4–5. doi:10.1152/japplphysiol.00448.2009.
Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354(3):270–82. doi:10.1056/NEJMra050776.
Acknowledgments
The authors have no conflicts of interest relevant to this review to declare. No specific funding sources were used to write this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Velders, M., Diel, P. How Sex Hormones Promote Skeletal Muscle Regeneration. Sports Med 43, 1089–1100 (2013). https://doi.org/10.1007/s40279-013-0081-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-013-0081-6