Abstract
Background
In 2014, lurasidone, an atypical antipsychotic, was approved for the treatment of schizophrenia in adults. It is an alternative treatment option to aripiprazole, and when compared with aripiprazole, lurasidone was associated with improved symptom reduction and reduced risk of weight gain and relapse. We conducted a cost-utility analysis of lurasidone versus aripiprazole from the perspective of healthcare services, using Scotland and Wales as specific case studies.
Methods
A 10-year Markov model, incorporating a 6-week acute phase and a maintenance phase across three health states (discontinuation, relapse, death) was constructed. Six-week probabilities of discontinuation and adverse events were based on a published independent mixed-treatment comparison; long-term risks of relapse and discontinuation were from an indirect comparison. Costs included drug therapy, relapse, and outpatient, primary and residential care. Costs and benefits were discounted at 3.5 %. Utility estimates were taken from published literature, and cost effectiveness was expressed as total 10-year incremental costs and quality-adjusted life-years (QALYs).
Results
Lurasidone yielded a cost saving of £3383 and an improvement of 0.005 QALYs versus aripiprazole, in Scotland. Deterministic sensitivity analysis demonstrated that results were sensitive to relapse rates, while probabilistic sensitivity analysis suggested that lurasidone had the highest expected net benefit at willingness-to-pay thresholds of £20,000–30,000 per QALY. The probability that lurasidone was a cost-effective treatment strategy was approximately 75 % at all willingness-to-pay thresholds, with similar results being obtained for the Welsh analysis.
Conclusions
Our analysis suggests that lurasidone would provide an effective, cost-saving alternative for the healthcare service in the treatment of adult patients with schizophrenia.
Similar content being viewed by others
Treatment of schizophrenia with atypical antipsychotics may be associated with weight gain and metabolic side effects. |
Lurasidone is a recently approved atypical antipsychotic for the treatment of schizophrenia in adults in Scotland. |
Lurasidone is associated with statistically significant improvements in efficacy and was generally well-tolerated in clinical studies when compared with other common atypical antipsychotics. |
Lurasidone is most likely to displace aripiprazole in patients with schizophrenia at risk of weight gain and/or metabolic disease. |
Lurasidone is likely to provide overall savings due to lower relapse rates and greater improvements in quality of life when compared with aripiprazole. |
1 Introduction
Schizophrenia is a chronic and disabling mental health condition resulting in progressive neurocognitive dysfunction, leading to alterations in perception, thoughts, mood and behaviour [1]. It has a lifetime risk of approximately 1 % and has a significant health, emotional and social impact on the patient, leading to social isolation, disability and dependence, unemployment and, in extreme cases, imprisonment and homelessness [2, 3]. The condition has a significant financial burden; in England, the total combined annual cost to society and the public sector was estimated to be £19 billion in 2010/11 [4]. The mainstay of current treatment for acute schizophrenic episodes, symptom reduction, and relapse prevention in patients with schizophrenia is antipsychotic medication [5]. It is recognised in numerous national and international guidelines that patients with schizophrenia should be treated with first- or second-line antipsychotics, and offered clozapine after prior failure of two antipsychotics [6–8]. The choice of antipsychotics should be based on a combination of treatment efficacy, tolerability, and patient and carer preferences [6, 7]. In the UK, current Scottish Intercollegiate Guidelines Network (SIGN) guidelines (131) recommend that olanzapine, risperidone or amisulpride should be prescribed for first-line treatment of patients with acute exacerbation or recurrence of schizophrenia, and for maintenance treatment [9].
Compounding this debilitating mental condition, comorbidities related to cardiovascular disease and metabolic disorders, such as diabetes, hypertension, metabolic syndrome, and obesity, are disproportionately prevalent among patients with schizophrenia [10]. Compared with the general population, patients with schizophrenia have almost twice the risk of metabolic syndrome (40.9 vs. 23.7 %, respectively) and diabetes (10.3 vs. 5.6 %, respectively) [11, 12], as well as an increased risk of cardiovascular disease-related mortality, with patients’ life expectancy reduced by an average of 15 years [13]. The prevalence of cardiovascular risk factors is also disproportionately high among patients with schizophrenia, of whom 58 % have dyslipidaemia, 45 % have hypertension and 15 % have abnormal fasting glucose, while 68 % are obese [14].
Although the presence of some modifiable cardiovascular disease risk factors, such as an increased sedentary lifestyle, may be specifically attributable to schizophrenia, a number of atypical antipsychotics have been associated with an increased risk of weight gain and other metabolic abnormalities [15–17]. These adverse effects frequently lead to discontinuation and/or cycling between different therapies [18–21]. Schizophrenia remains one of the most challenging disorders to treat due to a number of factors, including heterogeneity of presentation and patient response to treatment, disease-related risk of morbidity and mortality, and treatment-emergent adverse effects such as weight gain [22, 23]. For patients who are at risk of, or concerned about, weight gain, aripiprazole, haloperidol or amisulpride are recommended in SIGN guideline 131. This is supported by current National Institute for Health and Care Excellence (NICE) guidelines (CG178), which recommend that the potential risk of treatment-emergent weight gain should be considered when making treatment choices [5].
In January 2014, lurasidone, a new atypical antipsychotic, obtained marketing authorisation in Europe for the treatment of schizophrenia in adults [24]. In the UK, lurasidone has received positive recommendations for use by the Scottish Medicines Consortium (SMC) in Scotland “as an alternative treatment option in patients in whom it is important to avoid weight gain and metabolic adverse effects” and by the All Wales Medicines Strategy Group (AWMSG) as an option for use in adults aged 18 years and over [25, 26]. In five phase II and III, 12-month, double-blind, head-to-head studies, lurasidone was associated with significant improvements in symptom reduction and minimal changes in weight, body mass index, and metabolic outcomes versus placebo [27–31]. In studies where patients switched from a previous atypical antipsychotic to lurasidone, lurasidone was associated with improvements in weight and lipid levels, and demonstrated a low rate of treatment failure and high rate of study completion [32, 33]. When indirectly compared with other studies that have evaluated the efficacy and safety profile of atypical antipsychotics, such as aripiprazole, olanzapine, and quetiapine, lurasidone is associated with significant improvements in terms of weight gain, metabolic outcomes, relapse rates, hospitalisations, and rates of all-cause discontinuation [34–36].
While the clinical effectiveness of lurasidone in the treatment of schizophrenia has been demonstrated, the cost effectiveness of lurasidone versus alternative therapies remains to be established. We developed a model to evaluate the cost utility of introducing lurasidone as a treatment option for adult patients with schizophrenia from the perspective of healthcare services. In this study, we focus on Scotland and Wales as specific case studies in light of the recent SMC and AWMSG recommendations. These case studies compared the cost effectiveness of lurasidone versus aripiprazole as lurasidone is likely to replace aripiprazole as a treatment option for patients with schizophrenia.
2 Methods
2.1 Model Overview
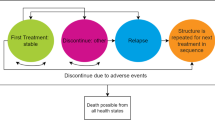
To reflect the chronic nature of the disease, a Markov model was constructed in Microsoft Excel (Microsoft Corporation, Redmond, WA, USA) (Fig. 1) to estimate the effectiveness (relapse, discontinuations, side effects and mortality) and costs for adult patients with schizophrenia. In line with previous economic evaluations [27, 30, 37], this cost-utility model assumes that treatment is initiated in a population with acute schizophrenia (acute phase), who then continue into a maintenance phase following disease stabilisation. In line with other models, a 10-year time horizon was used so that longer-term differences between treatments could be considered, and a discount rate of 3.5 % was applied to both costs and benefits [7, 38].
The model compares two alternative treatment sequences. For Scotland, current SIGN guidelines [9] state that “clozapine should be offered to service users who have treatment-resistant schizophrenia”, with treatment-resistant schizophrenia defined as “… failure to respond to an adequate trial of two different antipsychotics”. Based on this guidance, simplified treatment sequences were constructed. The first strategy consisted of lurasidone, followed by amisulpride, clozapine and, finally, an augmented clozapine strategy. The second differed from the first therapy in sequence only, which was aripiprazole.
Patients enter the model in an acute phase of relapse undergoing trials of antipsychotic agents (‘non-stable/Rx trial’ health state). Patients who have not discontinued treatment by week 6 are assumed to enter the ‘stable/adherent’ disease state—the maintenance phase—and are assumed initially to be on treatment. Those who have discontinued treatment at week 6 for any reason are assumed to switch therapy at this point and re-enter the non-stable/Rx trial health state to continue the process of trialling alternative antipsychotic agents. Patients may also die from any health state within the model. The 6-week endpoint for the acute phase of the model, and ongoing cycle length in the Markov model, was chosen to be consistent with the short-term studies of lurasidone [27, 37].
Individuals in the ‘stable/adherent’ health state in the maintenance phase are further subject to risks of all-cause discontinuation, relapse and death. Individuals discontinuing treatment in the maintenance phase are assumed to receive no therapy, and reside in the ‘stable/non-adherent’ health state until the onset of relapse, at which point they enter the ‘relapse’ health state. Relapse is considered to be treated either in an inpatient setting or at home, with treatment administered via the crisis resolution home treatment teams (CRHTTs), and patients who relapse are assumed to discontinue current therapy and switch to the next therapy in the sequence.
Reductions in health-related quality of life (HRQoL), as well as costs associated with weight gain (defined as a ≥7 % change in weight), presence of extrapyramidal symptoms (EPS) and diabetes, were applied, as experienced by patients in the model. Weight gain was assumed to persist while on treatment; EPS was assumed to persist for 3 months from the start of treatment, in line with the economic evaluation in NICE CG82, and incurred a one-off HRQoL decrement and cost; diabetes incidence occurred cumulatively over time from any state.
The main outcome measure of the analysis was the incremental cost-effectiveness ratio (ICER) for lurasidone versus aripiprazole, reported as cost per quality-adjusted life-year (QALY) gained. The electronic model has previously been reviewed by economists from UK national health technology assessment bodies [26, 39], and all clinical data and the model design were validated by an independent expert advisory board comprising nine clinicians in the UK.
2.2 Data Used in the Model
2.2.1 Clinical Efficacy
A 2013 independent systematic review and mixed treatment comparison (MTC) of atypical antipsychotics by Leucht et al. [34], including lurasidone and aripiprazole versus placebo, was used to inform estimates of short-term efficacy (probability of all-cause discontinuation) in the acute phase. Since the systematic review and MTC considered the relative effectiveness of lurasidone and aripiprazole versus placebo, it was necessary to establish an absolute placebo effect in order to estimate absolute effects for these therapies [27]. Model data inputs for all-cause discontinuation, EPS and weight gain for the acute phase are shown in Table 1, and a summary of all model input data is provided in Online Resource 1. Weight gain was considered clinically relevant if the patient experienced ≥7 % change in weight (measured in kilograms) from baseline. The independent MTC meta-analysis did not report long-term clinical outcomes, and no other comparative clinical data were available for lurasidone versus aripiprazole. Therefore, for the maintenance phase of the model, long-term risks of relapse and all-cause discontinuation for lurasidone were taken from a 12-month, randomised, double-blind, active-controlled study versus quetiapine [37]. To inform aripiprazole data, the quetiapine arm of the lurasidone trial was then compared with aripiprazole via an adjusted indirect comparison (via the Bucher method using olanzapine as the common comparator [40]), with relapse data taken from a 52-week, open-label extension to a 26-week comparison of aripiprazole with olanzapine [41], and from a 12-month, open-label extension study of quetiapine versus olanzapine [42]. This approach ensures that the relative effect of aripiprazole versus lurasidone can be calculated by discounting for the effect of the common comparator, quetiapine. To clarify, the adjusted indirect comparison of aripiprazole (A) computed an effect relative to quetiapine (B) by comparing aripiprazole (A) versus olanzapine (C) and quetiapine (B) versus olanzapine (C). In the absence of a common definition of relapse available across studies, all-cause hospitalisation was considered a proxy for relapse in the estimation of relative effects. We believe it is reasonable to consider relative treatment effects for all-cause hospitalisation as a proxy for relative treatment effect for relapse since hospitalisation is one of the variables measuring the composite endpoint ‘relapse’ in all clinical trials. For example, the definition of relapse provided by Loebel et al. [37] is “… the earliest occurrence of any of the following 3 criteria: (1) worsening of ≥30% in the PANSS total score from Day 42 of the initial acute treatment study and a CGI-S ≥3; (2) re-hospitalisation for worsening of psychosis; or (3) emergence of suicidal ideation, homicidal ideation and/or risk of harm”.
While the cause is unknown, the prevalence of diabetes in patients with schizophrenia ranges from 11.3 to 22.3 %, and therefore the risk of developing diabetes was included in the model [43–45]. To include the effect of diabetes in the current analysis, an approach similar to that of the NICE CG82 was used. The relative effect of developing diabetes was equal to the relative effect of experiencing weight gain. Cardiovascular events were not considered since including them would potentially lead to double-counting of the consequences of diabetes.
Mortality was based on published life tables of the general population, and adjusted to reflect the increased risk of mortality in patients with schizophrenia [46].
In the acute phase, patients cycled through a number of treatment regimens until they reached a stable disease state. The efficacy and safety data of subsequent therapies (amisulpride, clozapine, and augmented clozapine) were taken from Leucht et al. [34]. Data for augmented clozapine were assumed to equal the data for clozapine. In the absence of data, the risk of relapse and discontinuation versus quetiapine were assumed to be equal to quetiapine in the maintenance phase; the risk of relapse and discontinuation were assumed to remain constant throughout subsequent lines of therapy.
2.3 Health-State Utilities
A systematic review of health state utility values and HRQoL evidence in schizophrenia was performed. Electronic database searches were undertaken in November 2012, and conferences were searched between 2010 and 2012. Of the identified literature, those that were used in the development of the NICE guidelines were deemed to be the most appropriate to a UK clinical setting and were subsequently used to inform model estimates. To consider the impact of schizophrenia on patient HRQoL, utility scores reported in NICE CG82 and Lenert et al. were applied to patients in the stable and relapse health states [7, 47]. Lenert et al. derived utility weights using a convenience sample of the general population employing a standard gamble approach [47]. Disutilities associated with clinically relevant weight gain and EPS (expressed as percentage reductions in the utility score for stable disease) were taken from the same sources. Disutility for diabetes was not presented in NICE CG82; for this adverse event, an absolute utility decrement observed between schizophrenia with diabetes and stable schizophrenia of 0.15 was assumed from the values presented by Briggs et al. [48] (Table 2).
2.4 Costs
Cost assumptions were based on those in NICE CG82 [7] and were updated with current estimates or adjusted to 2013/14 costs using the Hospital Pay and Prices Index [49]. All costs were presented to an advisory board consisting of five psychiatrists and four pharmacists, and country-specific data were used where available. Costs included pharmacological therapies, adverse events, switching therapies, and outpatient, primary and community care costs related to general management of care for patients with schizophrenia, relapse, and residential care (Table 3). Individual costs for outpatient, primary and community care costs are reported in Online Resource 2.
List prices for pharmacological therapies were taken from the Monthly Index of Medical Specialities [50]. It was estimated that patients with schizophrenia receiving aripiprazole would require a once-daily dose of 15 mg based on UK prescribing data [21]. For lurasidone, the assumed once-daily dose was 40 or 80 mg, based on data used for the World Health Organization Anatomical Therapeutic Chemical application (data on file). Adverse event costs included those associated with EPS and weight gain. Treatment for patients with EPS was based on 100 % of patients receiving procyclidine (5 mg/day for 3 months) and one psychiatrist outpatient visit, while treatment for weight gain consisted of the cost of two general practitioner visits and three dietetic outpatient contacts based on 100 and 20 % of patients receiving these services, respectively. Outpatient, primary and community care costs were all adjusted to 6-week costs to fit the model cycle length. Cost of relapse was the combined cost of acute hospital admissions and CRHTT, assuming 30 and 70 % of patients receiving these services, respectively, and based on expert clinical opinion provided at the lurasidone advisory board. The mean duration of treatment for relapse was based on the duration reported in a review of crisis resolution home treatment services in Scotland [56], and the cost per case from that reported by Curtis [53]. Due to regional variations in the number of days of treatment, the cost per case was used and adjusted from £29,628 to £29,971 for the 2013/14 price year [49]. The 6-week cost of residential care was the total combined cost of patients being in private accommodation (77 %), sheltered housing (18 %), group housing (2 %) and long-term hospital care (3 %).
2.5 Sensitivity Analysis
Uncertainty surrounding model inputs was assessed through sensitivity analysis. In the univariate sensitivity analysis, all model parameters were systematically and independently varied over realistic ranges determined by (1) the 95 % confidence intervals surrounding the point estimates, or (2) sensible ranges of values where there was an absence of sampling uncertainty (±25 % of the point estimate). The net monetary benefit, assuming a recommended willingness-to-pay threshold of £20,000 per QALY [57–59], was recorded for lurasidone versus aripiprazole at the upper and lower parameter values, and was used to plot a Tornado diagram. Scenario analyses were also performed in which the values of key individual parameters were varied.
Joint parameter uncertainty was explored through probabilistic sensitivity analysis. All parameters were assigned distributions and varied jointly over 5000 simulations. Where possible, correlation between parameters was preserved by assuming multivariate normality. Results were plotted on a cost-effectiveness plane and cost-effectiveness acceptability curve.
3 Results
3.1 Base-Case Analysis
Table 4 presents the results of the base-case analysis of lurasidone versus aripiprazole over a 10-year time horizon. Lurasidone was associated with an overall cost saving of £3383 per patient and a modest increase of 0.005 QALYs, meaning that it was a dominant strategy versus generic aripiprazole (associated with reduced costs and increased benefits). Although total drug acquisition costs were £416 higher per patient treated with lurasidone (due to lower discontinuation modelled in the maintenance phase), these were offset by reduced costs of relapse (£3942), switching (£17), and adverse events (£50). Similar results were observed when data from Wales were used, where there was an overall cost saving of £3072 and an increase of 0.005 QALYs.
3.2 Univariate Sensitivity Analysis
Univariate sensitivity analysis revealed that the model parameters with the most impact on the cost effectiveness of lurasidone versus aripiprazole were those related to relapse rates. A negative net monetary benefit was generated (i.e. an ICER >£20,000 per QALY) when the hazard ratio of relapse for aripiprazole versus lurasidone was varied to the lower limit of the confidence interval. Despite being varied by a conservative ±25 % of the point estimate values, HRQoL values were not deemed as influencing factors for cost effectiveness. Figure 2 illustrates the results of the univariate analysis in the form of a Tornado diagram. Similar results from univariate sensitivity analyses were observed when the model was run using Wales-specific data.
3.3 Scenario Analysis
Results of the scenario analyses are presented in Table 5. At willingness-to-pay thresholds of £20,000 and £30,000 per QALY, lurasidone was a dominant strategy in all scenarios when compared with aripiprazole. The only scenario in which lurasidone was not considered cost effective was when no difference in relapse rates was assumed; in this scenario, lurasidone was associated with increased costs and fewer QALYs versus aripiprazole. However, this scenario should be interpreted with caution since relapses are driven largely by discontinuations due to the lack of efficacy and poor tolerability. It is therefore expected that if the relapse rates were equal between lurasidone and aripiprazole, then efficacy and tolerability may also be equal to or similar between the two drugs. In fact, it is implausible to have a scenario in which efficacy and tolerability were assumed to be different but have similar relapse rates. Note that similar results were observed when the model was run using Wales-specific data.
3.4 Probabilistic Sensitivity Analysis
The results from 5000 simulations are presented on a cost-effectiveness plane (Fig. 3) and cost-effectiveness acceptability curve (Fig. 4). Lurasidone was associated with the highest expected net benefit at all willingness-to-pay thresholds. The probability that lurasidone was the cost-effective strategy was approximately 75 % at all willingness-to-pay thresholds. Similar results were observed when the model was run using Wales-specific data, where the probability of lurasidone being a cost-effective strategy was approximately 70 % at all willingness-to-pay thresholds.
4 Discussion
We evaluated the cost effectiveness of lurasidone versus aripiprazole from the perspective of the National Health Service (NHS) and personal and social services in Scotland and Wales. The SIGN guidelines recommend that aripiprazole should be prescribed for patients with schizophrenia who are at risk of, or concerned about, weight gain; aripiprazole has come to represent the most widely prescribed treatment for this specific population. The economic evaluation suggests that treatment of adult patients with schizophrenia in Scotland and Wales with lurasidone is a cost-effective strategy when compared with aripiprazole. In the base-case analysis, lurasidone was associated with an overall 10-year cost saving of £3383 and an increase of 0.005 QALYs per patient. The saving in costs was primarily driven by reduced relapse, switching and adverse events. When the model was run to evaluate the cost utility of lurasidone in Wales, lurasidone was associated with an overall 10-year cost saving of £3072 and an increase of 0.005 QALYs. Similar to the case for the Scottish analysis, the saving in costs was driven by reduced relapse, switching, and adverse events.
Sensitivity analyses suggested that the cost effectiveness of lurasidone versus aripiprazole was only sensitive to relapse rates due to the high costs associated with relapse and all-cause discontinuation. Univariate analysis suggested that a negative net monetary benefit was generated only when relapse rates for lurasidone versus quetiapine were varied to the lower limits of the confidence interval. In addition, scenario analyses suggested that lurasidone was associated with increased costs and fewer QALYs when compared with aripiprazole when relapse rates were assumed to be equal for the two therapies. However, this analysis should be treated with caution. Probabilistic sensitivity analysis further supported the base-case results, demonstrating that lurasidone has a 75 and 70 % probability of being cost effective at all willingness-to-pay thresholds in Scotland and Wales, respectively.
There are several strengths to this economic analysis. Due to the chronic nature of schizophrenia and the long-term effects of the condition, the implementation of a Markov model and a 10-year time horizon allows the long-term assessment of cost effectiveness, and is in line with the model used in NICE CG82. Short-term relative efficacy data were taken directly from an independent, peer-reviewed systemic review and MTC [34, 37, 41]. Many cost assumptions were taken directly from NICE guidance [7], and all individual costs were updated with recent and/or country-specific data where possible. When this was not possible, all costs were adjusted to the 2013/14 price year using the Hospital Pay and Price index. Specifically, country-specific data were used to inform the cost of inpatient relapse, an important determining factor for cost effectiveness in this analysis. Additionally, all costs were independently verified and deemed appropriate by the lurasidone advisory board.
As with all economic models, there are a number of limitations to the analysis, primarily the reliance on an indirect comparison to inform long-term effects, discontinuation and relapse rates for aripiprazole. The comparison is based on a single study and relies on the assumption that the relative effect of hospitalisation is equivalent to the relative effect of relapse. However, retrospective real-world database studies from the US support the clinical effectiveness assumptions of lurasidone [60, 61] that formed the foundational assumptions of the cost-utility analysis. When compared with aripiprazole, lurasidone was associated with increased treatment adherence and mean length of continuous therapy [60]. In addition, patients who had taken lurasidone for 6 months had a significant reduction in the number of all-cause and mental health-related hospitalisations when compared with the 6 months prior to starting treatment with lurasidone [61]. It is difficult to determine the extent to which the results of our study are generalisable to other jurisdictions given the high degree of heterogeneity in the costs of schizophrenia management [62]. Notwithstanding these limitations, we are reasonably confident that the findings may be largely generalisable to other settings, given that relapse and hospitalisations are the driver of the economic model and that absence of relapse and hospitalisations are considered to be a measure of long-term effect in these chronically ill patients. The analysis adopts an NHS and personal social services perspective and does not therefore consider social care elements for patients, such as employment rates and reduced work-related productivity; thus, this analysis may underestimate the benefits of treatment with lurasidone [63].
5 Conclusions
Atypical antipsychotics may be associated with weight gain and metabolic side effects, leading to poor treatment adherence and discontinuation and/or cycling between treatment options [15–21]. Lurasidone is an atypical antipsychotic, recommended for use in adult patients with schizophrenia [25], and may be of particular value in patients who are at risk of, or concerned about, metabolic disease or weight gain.
When compared with aripiprazole, for patients at risk of, or concerned about, weight gain, lurasidone appears to be a dominant treatment strategy, resulting in a net monetary saving and an increase in QALYs. Sensitivity analyses indicate that the base-case results are robust, and lurasidone was only considered not cost-effective when very conservative relapse rates were considered. Therefore, from the perspective of the NHS and personal and social services in Scotland and Wales, lurasidone may represent a cost-effective treatment option for adult patients with schizophrenia, particularly when targeted at patients who are at risk of, or concerned about, weight gain and/or metabolic disease.
References
Picchioni MM, Murray RM. Schizophrenia. BMJ. 2007;335(7610):91–5.
Thornicroft G, Tansella M, Becker T, Knapp M, Leese M, Schene A, et al. The personal impact of schizophrenia in Europe. Schizophr Res. 2004;69(2–3):125–32.
Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421–7.
Andrew A, Knapp M, McCrone P, Parsonage M, Trachtenberg M. Effective interventions in schizophrenia. The economic case. A report prepared for the Schizophrenia Commission. London: Personal Social Services Research Unit, London School of Economics; 2012.
National Institute for Health and Care Excellence. Psychosis and schizophrenia in adults: treatment and management. NICE clinical guideline CG 178. London: National Institute for Health and Care Excellence; 2014.
Taylor D, Paton C, Kapur S. The Maudsley prescribing guidelines in psychiatry. Hoboken: Wiley Blackwell; 2012.
National Institute for Health and Care Excellence. Psychosis and schizophrenia: management. NICE clinical guideline CG 82. London: National Institute for Health and Care Excellence; 2009.
Gaebel W, Weinmann S, Sartorius N, Rutz W, McIntyre JS. Schizophrenia practice guidelines: international survey and comparison. Br J Psychiatry. 2005;187:248–55.
Scottish Intercollegiate Guidelines Network (SIGN). Management of schizophrenia. Edinburgh: Scottish Intercollegiate Guidelines Network; 2013. Available at: http://www.sign.ac.uk.
McDermott S, Moran R, Platt T, Isaac T, Wood H, Dasari S. Heart disease, schizophrenia, and affective psychoses: epidemiology of risk in primary care. Community Ment Health J. 2005;41(6):747–55.
Bresee LC, Majumdar SR, Patten SB, Johnson JA. Prevalence of cardiovascular risk factors and disease in people with schizophrenia: a population-based study. Schizophr Res. 2010;117(1):75–82.
McEvoy JP, Meyer JM, Goff DC, Nasrallah HA, Davis SM, Sullivan L, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res. 2005;80(1):19–32.
Kelly DL, McMahon RP, Liu F, Love RC, Wehring HJ, Shim JC, et al. Cardiovascular disease mortality in patients with chronic schizophrenia treated with clozapine: a retrospective cohort study. J Clin Psychiatry. 2010;71(3):304–11.
Kato MM, Currier MB, Villaverde O, Gonzalez-Blanco M. The relation between body fat distribution and cardiovascular risk factors in patients with schizophrenia: a cross-sectional pilot study. Prim Care Companion J Clin Psychiatry. 2005;7(3):115–8 (quiz 9–20).
Correll CU, Frederickson AM, Kane JM, Manu P. Metabolic syndrome and the risk of coronary heart disease in 367 patients treated with second-generation antipsychotic drugs. J Clin Psychiatry. 2006;67(4):575–83.
L’Italien GJ, Casey DE, Kan HJ, Carson WH, Marcus RN. Comparison of metabolic syndrome incidence among schizophrenia patients treated with aripiprazole versus olanzapine or placebo. J Clin Psychiatry. 2007;68(10):1510–6.
Sernyak MJ, Leslie DL, Alarcon RD, Losonczy MF, Rosenheck R. Association of diabetes mellitus with use of atypical neuroleptics in the treatment of schizophrenia. Am J Psychiatry. 2002;159(4):561–6.
American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. J Clin Psychiatry. 2004;65(2):267–72.
Lindenmayer JP, Czobor P, Volavka J, Citrome L, Sheitman B, McEvoy JP, et al. Changes in glucose and cholesterol levels in patients with schizophrenia treated with typical or atypical antipsychotics. Am J Psychiatry. 2003;160(2):290–6.
Mackin P, Bishop D, Watkinson H, Gallagher P, Ferrier IN. Metabolic disease and cardiovascular risk in people treated with antipsychotics in the community. Br J Psychiatry. 2007;191:23–9.
Nasrallah HA, Meyer JM, Goff DC, McEvoy JP, Davis SM, Stroup TS, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1–3):15–22.
Lehman AF, Lieberman JA, Dixon LB, McGlashan TH, Miller AL, Perkins DO, et al. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(2 Suppl):1–56.
Tandon R, Belmaker RH, Gattaz WF, Lopez-Ibor JJ Jr, Okasha A, Singh B, et al. World Psychiatric Association Pharmacopsychiatry Section statement on comparative effectiveness of antipsychotics in the treatment of schizophrenia. Schizophr Res. 2008;100(1–3):20–38.
European Medicines Agency. Summary of opinion: Latuda. Committee for Medicinal Products for Human Use (CHMP), EMA/CHMP/26583/2014. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion_-_Initial_authorisation/human/002713/WC500160099.pdf.
Scottish Medicines Consortium. Sunovion: SMC No. (994/14) 2014. Available at: https://www.scottishmedicines.org.uk/files/advice/lurasidone__Latuda__FINAL_Sept_2014_amended_15.09.14_for_website.pdf. Accessed 5 May 2015.
All Wales Medicines Strategy Group. Final appraisal recommendation (advice no: 0115, February 2015). Lurasidone (Latuda®) 18.5 mg, 37 mg and 74 mg film-coated tablets. 2015. Available at: http://www.awmsg.org/awmsgonline/app/appraisalinfo/1142. Accessed Sep 2015.
Loebel A, Cucchiaro J, Sarma K, Xu L, Hsu C, Kalali AH, et al. Efficacy and safety of lurasidone 80 mg/day and 160 mg/day in the treatment of schizophrenia: a randomized, double-blind, placebo- and active-controlled trial. Schizophr Res. 2013;145(1–3):101–9.
Meltzer HY, Cucchiaro J, Silva R, Ogasa M, Phillips D, Xu J, et al. Lurasidone in the treatment of schizophrenia: a randomized, double-blind, placebo- and olanzapine-controlled study. Am J Psychiatry. 2011;168(9):957–67.
Nakamura M, Ogasa M, Guarino J, Phillips D, Severs J, Cucchiaro J, et al. Lurasidone in the treatment of acute schizophrenia: a double-blind, placebo-controlled trial. J Clin Psychiatry. 2009;70(6):829–36.
Nasrallah HA, Silva R, Phillips D, Cucchiaro J, Hsu J, Xu J, et al. Lurasidone for the treatment of acutely psychotic patients with schizophrenia: a 6-week, randomized, placebo-controlled study. J Psychiatr Res. 2013;47(5):670–7.
Ogasa M, Kimura T, Nakamura M, Guarino J. Lurasidone in the treatment of schizophrenia: a 6-week, placebo-controlled study. Psychopharmacology (Berl). 2013;225(3):519–30.
Stahl SM, Cucchiaro J, Simonelli D, Hsu J, Pikalov A, Loebel A. Effectiveness of lurasidone for patients with schizophrenia following 6 weeks of acute treatment with lurasidone, olanzapine, or placebo: a 6-month, open-label, extension study. J Clin Psychiatry. 2013;74(5):507–15.
McEvoy JP, Citrome L, Hernandez D, Cucchiaro J, Hsu J, Pikalov A, et al. Effectiveness of lurasidone in patients with schizophrenia or schizoaffective disorder switched from other antipsychotics: a randomized, 6-week, open-label study. J Clin Psychiatry. 2013;74(2):170–9.
Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951–62.
Rajagopalan K, Hassan M, O’Day K, Meyer K, Grossman F. Cost-effectiveness of lurasidone vs aripiprazole among patients with schizophrenia who have previously failed on an atypical antipsychotic: an indirect comparison of outcomes from clinical trial data. J Med Econ. 2013;16(7):951–61.
Rajagopalan K, O’Day K, Meyer K, Pikalov A, Loebel A (eds). Discontinuation rates among atypical antipsychotics for schizophrenia: an indirect treatment comparison. Academy of Managed Care Pharmacy Educational Conference, 3–5 Oct 2012, Cincinnati (OH).
Loebel A, Cucchiaro J, Xu J, Sarma K, Pikalov A, Kane JM. Effectiveness of lurasidone vs. quetiapine XR for relapse prevention in schizophrenia: a 12-month, double-blind, noninferiority study. Schizophr Res. 2013;147(1):95–102.
Davies A, Vardeva K, Loze JY, L’Italien GJ, Sennfalt K, Van Baardewijk M. Cost-effectiveness of atypical antipsychotics for the management of schizophrenia in the UK. Curr Med Res Opin. 2008;24(11):3275–85.
Scottish Medicines Consortium. SMC advice for lurasidone (Latuda). 2014. Available at: https://www.scottishmedicines.org.uk/SMC_Advice/Advice/994_14_lurasidone_Latuda/lurasidone_Latuda. Accessed 26 Oct 2015.
Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50(6):683–91.
Chrzanowski WK, Marcus RN, Torbeyns A, Nyilas M, McQuade RD. Effectiveness of long-term aripiprazole therapy in patients with acutely relapsing or chronic, stable schizophrenia: a 52-week, open-label comparison with olanzapine. Psychopharmacology (Berl). 2006;189(2):259–66.
Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209–23.
Woo V, Harris SB, Houlden RL, on behalf of the Clinical and Scientific Section, Canadian Diabetes Association. Canadian Diabetes Association Position Paper: antipsychotic medications and associated risks of weight gain and diabetes. Can. J Diabetes. 2005;29(2):111–2.
Zhang R, Hao W, Pan M, Wang C, Zhang X, da Chen C, et al. The prevalence and clinical-demographic correlates of diabetes mellitus in chronic schizophrenic patients receiving clozapine. Hum Psychopharmacol. 2011;26(6):392–6.
Schoepf D, Potluri R, Uppal H, Natalwala A, Narendran P, Heun R. Type-2 diabetes mellitus in schizophrenia: increased prevalence and major risk factor of excess mortality in a naturalistic 7-year follow-up. Eur Psychiatry. 2012;27(1):33–42.
Brown S, Kim M, Mitchell C, Inskip H. Twenty-five year mortality of a community cohort with schizophrenia. Br J Psychiatry. 2010;196(2):116–21.
Lenert LA, Sturley AP, Rapaport MH, Chavez S, Mohr PE, Rupnow M. Public preferences for health states with schizophrenia and a mapping function to estimate utilities from positive and negative symptom scale scores. Schizophr Res. 2004;71(1):155–65.
Briggs A, Wild D, Lees M, Reaney M, Dursun S, Parry D, et al. Impact of schizophrenia and schizophrenia treatment-related adverse events on quality of life: direct utility elicitation. Health Qual Life Outcomes. 2008;6:105.
Curtis L. Unit costs of health and social care 2012. Canterbury: Personal Social Services Research Unit, University of Kent; 2012.
Monthly Index of Medical Specialities. Available at: http://www.mims.co.uk/. Accessed 25 Sep 2015.
Cegedim Strategic Data. Neuroleptics report 1. MAT. Data on file; 2014.
Information Services Division. Specialty group costs and activity: consultant outpatients, by specialty, by board. R044X: 2013. Available at: http://www.isdscotland.org/Health-Topics/Finance/Costbook/Speciality-Costs/Long-Stay-Specialties.asp.
Curtis L. Unit costs of health and social care 2014. Canterbury: Personal Social Services Research Unit, University of Kent; 2014.
Department of Health. Reference Cost Collection: National schedule of reference costs—year 2013–14. NHS Trusts and NHS Foundation Trusts. Department of Health; 2014.
Information Services Division. Speciality group costs: inpatients in long stay specialties R040LSX. 2014. Available at: http://www.isdscotland.org/Health-Topics/Finance/Costbook/Speciality-Costs/Long-Stay-Specialties.asp.
Williamson P, Marshall L. A review of crisis resolution home treatment services in Scotland. Version 4, Nov 2011. Available at: http://www.qihub.scot.nhs.uk/media/264761/crisis_resolution_home_treatment_report%20final%20november.pdf.
National Institute for Health and Care Excellence. Guide to the methods of technology appraisal 2013. Available at: http://www.nice.org.uk/article/pmg9/resources/non-guidance-guide-to-the-methods-of-technology-appraisal-2013-pdf. Accessed 30 Sep 15.
All Wales Medicines Strategy Group. Summary guidelines for appraising medicines. 2012. Available at: http://www.awmsg.org/docs/awmsg/appraisaldocs/inforandforms/AWMSG%20summary%20guidelines%20for%20appraising%20medicines.pdf. Accessed 30 Sep 15.
Scottish Medicines Consortium. A guide to quality adjusted life years. 2011. Available at: http://www.scottishmedicines.org.uk/About_SMC/Policy_statements/A_Guide_to_Quality_Adjusted_Life_Years. Accessed 30 Sep 15.
Hassan M, Wade S, Meyer N, Pikalov A, Loebel A, Rajagopalan K. Comparison of treatment adherence among new-start patients on lurasidone vs other atypical antipsychotics: results from a multi-state Medicaid population among adults with schizophrenia. Poster presented at the US Psychiatric and Mental Health Congress (USPMHC), 30 Sep–3 Oct 2013, Las Vegas (NV).
Hassan M, Wade S, Meyer N, Pikalov A, Loebel A, Rajagopalan K. Inpatient admissions among schizophrenia patients before and after initiating lurasidone in a multi-state Medicaid population. Poster presented at the US Psychiatric and Mental Health Congress (USPMHC), 30 Sep–3 Oct 2013, Las Vegas (NV).
Essock S, Frisman L, Covell N. The economics of the treatment of schizophrenia. In: Davis KL, Charney D, Coyle JT, Nemeroff C, editors. Neuropsychopharmacology: the fifth generation of progress. Brentwood: American College of Neuropsychopharmacology; 2002. p. 809–18.
Marwaha S, Johnson S, Bebbington P, Stafford M, Angermeyer MC, Brugha T, et al. Rates and correlates of employment in people with schizophrenia in the UK, France and Germany. Br J Psychiatry. 2007;191(1):30–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Krithika Rajagopalan and Dr. Antony Loebel are paid employees of Sunovion Pharmaceuticals, Inc., and David Trueman, Lydia Crowe and Daniel Squirrell are paid employees of DRG Abacus. DRG Abacus conducted the research, and received consulting fees from Sunovion Pharmaceuticals Inc.
Funding
This research was funded by Sunovion Pharmaceuticals, Inc.
Author contributions
Krithika Rajagopalan conceived and contributed to the design of the evaluation. Antony Loebel led the clinical trial that provided the data source for the model, and provided support to Krithika Rajagopalan by ensuring clinical accuracy in designing the treatment pathway for economic evaluation. David Trueman developed the economic model, contributed to the design, and performed all analyses. Lydia Crowe contributed to the design of the analysis. Daniel Squirrell contributed to the design and analysis of the model and wrote the manuscript. All authors played a role in the review of the analysis, interpretation of the results, and reviewed and recommended revisions to the final submitted manuscript to ensure accuracy and fair balance.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Rajagopalan, K., Trueman, D., Crowe, L. et al. Cost-Utility Analysis of Lurasidone Versus Aripiprazole in Adults with Schizophrenia. PharmacoEconomics 34, 709–721 (2016). https://doi.org/10.1007/s40273-016-0405-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-016-0405-0