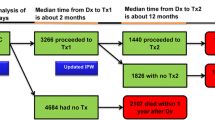
Abstract
Background
Chemotherapy prolongs survival for stage III colon cancer patients but community-level evidence on the effectiveness and cost effectiveness of treatment for elderly patients is limited. Comparisons were between patients receiving no chemotherapy, 5-fluorouracil (5-FU), and FOLFOX (5-FU + oxaliplatin).
Methods
A retrospective cohort study was conducted using the Surveillance Epidemiology, and End Results (SEER)–Medicare linked database. Patients (≥65 years) with American Joint Committee on Cancer stage III colon cancer at diagnosis in 2004–2009 were identified. The 3-way propensity score matched sample included 3,534 patients. Effectiveness was measured in life-years and quality-adjusted life-years (QALYs). Medicare costs (2010 US dollars) were estimated from diagnosis until death or end of study.
Results
FOLFOX patients experienced 6.06 median life-years and 4.73 QALYs. Patients on 5-FU had 5.75 median life-years and 4.50 median QALYs, compared to 3.42 and 2.51, respectively, for the no chemotherapy patients. Average total healthcare costs ranged from US$85,422 for no chemotherapy to US$168,628 for FOLFOX. Incremental cost-effectiveness ratios (ICER) for 5-FU versus no chemotherapy were US$17,131 per life-year gained and US$20,058 per QALY gained. ICERs for FOLFOX versus 5-FU were US$139,646 per life-year gained and US$188,218 per QALY gained. Results appear to be sensitive to age, suggesting that FOLFOX performs better for patients 65–69 and 80+ years old while 5-FU appears most effective and cost effective for the age groups 70–74 and 75–79 years.
Conclusion
FOLFOX appears more effective and cost effective than other strategies for colon cancer treatment of older patients. Results were sensitive to age, with ICERs exhibiting a U-shaped pattern.

Similar content being viewed by others
References
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–717.
Cole BF, Gelber RD, Gelber S, Coates AS, Goldhirsch A. Polychemotherapy for early breast cancer: an overview of the randomised clinical trials with quality-adjusted survival analysis. Lancet. 2001;358(9278):277–86.
Polychemotherapy for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1998;352(9132):930–42.
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Clarke M, Coates AS, Darby SC, Davies C, Gelber RD, et al. Adjuvant chemotherapy in oestrogen-receptor-poor breast cancer: patient-level meta-analysis of randomised trials. Lancet. 2008;371(9606):29–40.
Sargent DJ, Goldberg RM, Jacobson SD, Macdonald JS, Labianca R, Haller DG, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med. 2001;345(15):1091–7.
Goldberg RM, Tabah-Fisch I, Bleiberg H, de Gramont A, Tournigand C, Andre T, et al. Pooled analysis of safety and efficacy of oxaliplatin plus fluorouracil/leucovorin administered bimonthly in elderly patients with colorectal cancer. J Clin Oncol. 2006;24(25):4085–91.
NIH Consensus Conference. Adjuvant therapy for breast cancer. J Natl Cancer Inst. 2001;93:979–89.
Hutchins LF, Unger JM, Crowley JJ, Coltman CA Jr, Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341(27):2061–7.
Lewis JH, Kilgore ML, Goldman DP, Trimble EL, Kaplan R, Montello MJ, et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol. 2003;21(7):1383–9.
Grothey A, Sargent DJ. FOLFOX for stage II colon cancer? A commentary on the recent FDA approval of oxaliplatin for adjuvant therapy of stage III colon cancer. J Clin Oncol. 2005;23(15):3311–3.
Food and Drug Administration. Eloxatin: new or modified indication. Washington, DC: US Food and Drug Administration; 2004.
Hillner BE, Schrag D, Sargent DJ, Fuchs CS, Goldberg RM. Cost-effectiveness projections of oxaliplatin and infusional fluorouracil versus irinotecan and bolus fluorouracil in first-line therapy for metastatic colorectal carcinoma. Cancer. 2005;104(9):1871–84.
Mullins CD, Hsiao F, Onukwugha E, Pandya NB, Hanna N. Comparative and cost-effectiveness of oxaliplatin-based or irinotecan-based regimens compared with 5-fluorouracil/leucovorin alone among US elderly stage IV colon cancer patients. Cancer. 2012;118(12):3173–81.
Pandor A, Eggington S, Paisley S, Tappenden P, Sutcliffe PA. The clinical and cost-effectiveness of oxaliplatin and capecitabine for the adjuvant treatment of colon cancer: systematic review and economic evaluation. Health Technol Assess. 2006;10(41):1–204.
Attard C, Maroun J, Alloul K, Grima D, Bernard L. Cost-effectiveness of oxaliplatin in the adjuvant treatment of colon cancer in Canada. Curr Oncol. 2010;17(1):17.
Etzioni R, Ramsey SD, Berry K, Brown M. The impact of including future medical care costs when estimating the costs attributable to a disease: a colorectal cancer case study. Health Econ. 2001;10(3):245–56.
Brown ML, Riley GF, Potosky AL, Etzioni RD. Obtaining long-term disease specific costs of care: application to Medicare enrollees diagnosed with colorectal cancer. Med Care. 1999;37(12):1249–59.
Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(8):IV-3–-18.
Rassen JA, Doherty M, Huang W, Schneeweiss S. Pharmacoepidemiology toolbox; 2013. http://www.drugepi.org/wp-content/uploads/2013/10/Using_the_Pharmacoepi_Toolbox_in_SAS_2.4.15.pdf. Accessed 20 Feb 2014.
Best JH, Garrison LP, Hollingworth W, Ramsey SD, Veenstra DL. Preference values associated with stage III colon cancer and adjuvant chemotherapy. Qual Life Res. 2010;19(3):391–400.
Ramsey SD, Andersen MR, Etzioni R, Moinpour C, Peacock S, Potosky A, et al. Quality of life in survivors of colorectal carcinoma. Cancer. 2000;88(6):1294–303.
Allen E, Nicolaidis C, Helfand M. The evaluation of rectal bleeding in adults. J Gen Intern Med. 2005;20(1):81–90.
Sail KR, Franzini L, Lairson DR, Du XL. Clinical and economic outcomes associated with adjuvant chemotherapy in elderly patients with early stage operable breast cancer. Value Health. 2012;15(1):72–80.
Havrilesky LJ, Broadwater G, Davis DM, Nolte KC, Barnett JC, Myers ER, et al. Determination of quality of life-related utilities for health states relevant to ovarian cancer diagnosis and treatment. Gynecol Oncol. 2009;113(2):216–20.
Earle CC, Nattinger AB, Potosky AL, Lang K, Mallick R, Berger M, et al. Identifying cancer relapse using SEER-Medicare data. Med Care. 2002;40(8):IV-75–81.
Gray AM, Clarke PM, Wolstenholme JL, Wordsworth S. Applied methods of cost-effectiveness analysis in healthcare. Oxford: Oxford University Press; 2010.
Wu B, Dong B, Xu Y, Zhang Q, Shen J, Chen H, et al. Economic evaluation of first-line treatments for metastatic renal cell carcinoma: a cost-effectiveness analysis in a health resource-limited setting. PLoS One. 2012;7(3):e32530.
Zhu H, Xia X, Yu C, Adnan A, Liu S, Du Y. Application of Weibull model for survival of patients with gastric cancer. BMC Gastroenterol. 2011;11(1):1.
Brown ML, Riley GF, Schussler N, Etzioni R. Estimating health care costs related to cancer treatment from SEER-Medicare data. Med Care. 2002;40(8):IV-104–17.
Medical care—consumer price index; 2013. http://data.bls.gov/timeseries/CUUR0000SAM?output_view=pct_12mths. Accessed 20 Feb 2014.
Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424.
Chitnis AS, Aparasu RR, Chen H, Johnson ML. Effect of certain angiotensin-converting enzyme inhibitors on mortality in heart failure: a multiple-propensity analysis. Res Soc Admin Pharm. 2012;8(2):145–56.
Schrag D, Cramer LD, Bach PB, Begg CB. Age and adjuvant chemotherapy use after surgery for stage III colon cancer. J Natl Cancer Inst. 2001;93(11):850–7.
Panchal JM, Lairson DR, Chan W, Du XL. Geographic variation and sociodemographic disparity in the use of oxaliplatin-containing chemotherapy in patients with stage III colon cancer. Clin Colorectal Cancer. 2013;12(2):113–21.
Aballéa S, Chancellor JV, Raikou M, Drummond MF, Weinstein MC, Jourdan S, et al. Cost-effectiveness analysis of oxaliplatin compared with 5-fluorouracil/leucovorin in adjuvant treatment of stage III colon cancer in the US. Cancer. 2007;109(6):1082–9.
Howard DH, Kauh J, Lipscomb J. The value of new chemotherapeutic agents for metastatic colorectal cancer. Arch Intern Med. 2010;170(6):537.
Bradburn M, Clark T, Love S, Altman D. Survival analysis part II: multivariate data analysis–an introduction to concepts and methods. Br J Cancer. 2003;89(3):431.
Bradburn M, Clark T, Love S, Altman D. Survival analysis part III: multivariate data analysis—choosing a model and assessing its adequacy and fit. Br J Cancer. 2003;89(4):605.
Malin JL. Wrestling with the high price of cancer care: should we control costs by individuals’ ability to pay or society’s willingness to pay? J Clin Oncol. 2010;28(20):3212–4.
Mason A, Drummond M, Ramsey S, Campbell J, Raisch D. Comparison of anticancer drug coverage decisions in the United States and United Kingdom: does the evidence support the rhetoric? J Clin Oncol. 2010;28(20):3234–8.
American College of Physicians. Information on cost-effectiveness: an essential product of a national comparative effectiveness program. Ann Intern Med. 2008;148:956–61.
Murden RA, Seiber EE. How can cost-effectiveness information help control unsustainable growth in U.S. health care spending? [letter]. Ann Intern Med. 2009;150(1):58.
Acknowledgments
This study was supported by a grant from the Agency for Healthcare Research and Quality (R01-HS018956) and in part by a grant from Cancer Prevention Research Institute of Texas (RP130051). The authors have no conflicts of interest to declare. Authors D.L., R.P., and X.D. were primarily responsible for study design, data analysis, and manuscript writing. Authors J.C. and W.C. were responsible for study design, interpretation of data, and critical review of the manuscript. D.L. is the overall guarantor for the content.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lairson, D.R., Parikh, R.C., Cormier, J.N. et al. Cost–Utility Analysis of Chemotherapy Regimens in Elderly Patients with Stage III Colon Cancer. PharmacoEconomics 32, 1005–1013 (2014). https://doi.org/10.1007/s40273-014-0180-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-014-0180-8