Abstract
Objective
In the current study, we propose an approach for selection of a model that is transferable to a specific decision-making context (in this case, the Netherlands), using the case of rheumatoid arthritis (RA). The objectives of this study were (a) to perform a systematic literature review to identify existing health economic evaluation models for economic evaluation of disease-modifying antirheumatic drugs (DMARDs) in RA; and (b) to test the appropriateness of a stepwise model-selection process.
Methods
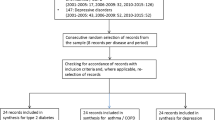
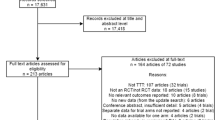
First, we searched Medline and Embase to identify relevant studies in the English language, published between 1 January 2002 and 31 August 2012. From the included studies, all unique models were identified. Second, we applied a multi-step approach to model selection. Models that did not meet all minimal methodological and structural requirements based on the Outcome Measures in Rheumatology (OMERACT) criteria were excluded. Next, models were assessed on the basis of their fit when transferred to the Dutch health care setting. The criteria for model fit were transferability factors, as published by Welte et al., after exclusion of those that were deemed transferable by simple adaptation. Finally, the remaining models underwent a general quality check using the Philips checklist. Models showing good fit and high quality were considered to be transferable to the Dutch health care setting, using simple adaptation.
Results
The systematic literature search identified 498 articles, which included 33 unique health economic evaluation models. Only six models passed the minimal methodological and structural requirements. Two of these models had an imperfect transferability fit to the Dutch health care setting, according to the Welte method. The remaining four models were, according to the Philips method, of good quality and were expected to be transferable by a simple adaptation.
Conclusion
This study introduces a stepwise approach for selecting health economic evaluation models that are transferable by a simple adaptation. The approach seems feasible and can be applied in various therapeutic areas, provided that the minimal methodological and structural requirements are defined accordingly. Availability of health economic evaluation models coupled with structured model selection could improve the efficiency, quality and comparability of health economic research.

Similar content being viewed by others
Notes
Some models use survival statistics based on observational data to predict the subjects’ time on treatment. We also considered this a suitable approach for modelling health state transitions (i.e. treatment discontinuation).
References
Sculpher MJ, Pang FS, Manca A, Drummond MF, Golder S, Urdahl H, et al. Generalisability in economic evaluation studies in healthcare: a review and case studies. Health Technol Assess. 2004;8(49):iii,iv, 1–192.
Drummond M, Barbieri M, Cook J, Glick HA, Lis J, Malik F, et al. Transferability of economic evaluations across jurisdictions: ISPOR Good Research Practices Task Force report. Value Health. 2009;12(4):409–18.
Goeree R, He J, O’Reilly D, Tarride JE, Xie F, Lim M, et al. Transferability of health technology assessments and economic evaluations: a systematic review of approaches for assessment and application. Clinicoecon Outcomes Res. 2011;3:89–104.
Welte R, Feenstra T, Jager H, Leidl R. A decision chart for assessing and improving the transferability of economic evaluation results between countries. Pharmacoeconomics. 2004;22(13):857–76.
Alamanos Y, Drosos AA. Epidemiology of adult rheumatoid arthritis. Autoimmun Rev. 2005;4(3):130–6.
Eriksson JK, Neovius M, Ernestam S, Lindblad S, Simard JF, Askling J. Incidence of rheumatoid arthritis in Sweden—a nationwide population-based assessment of incidence, its determinants, and treatment penetration. Arthritis Care Res (Hoboken). 2013;65:870–8.
Strand V, Singh JA. Improved health-related quality of life with effective disease-modifying antirheumatic drugs: evidence from randomized controlled trials. Am J Manag Care. 2008;14(4):234–54.
Fautrel B. Economic benefits of optimizing anchor therapy for rheumatoid arthritis. Rheumatology (Oxford). 2012 Jun;51 Suppl 4:iv21–6.
Maetzel A, Tugwell P, Boers M, Guillemin F, Coyle D, Drummond M, et al. Economic evaluation of programs or interventions in the management of rheumatoid arthritis: defining a consensus-based reference case. J Rheumatol. 2003;30(4):891–6.
Bansback N, Ara R, Karnon J, Anis A. Economic evaluations in rheumatoid arthritis: a critical review of measures used to define health states. Pharmacoeconomics. 2008;26(5):395–408.
Bansback NJ, Regier DA, Ara R, Brennan A, Shojania K, Esdaile JM, et al. An overview of economic evaluations for drugs used in rheumatoid arthritis: focus on tumour necrosis factor-alpha antagonists. Drugs. 2005;65(4):473–96.
Briggs AH, Weinstein MC, Fenwick EA, Karnon J, Sculpher MJ, Paltiel AD, et al. Model parameter estimation and uncertainty: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force—6. Value Health. 2012;15(6):835–42.
Philips Z, Bojke L, Sculpher M, Claxton K, Golder S. Good practice guidelines for decision-analytic modelling in health technology assessment: a review and consolidation of quality assessment. Pharmacoeconomics. 2006;24(4):355–71.
Cimmino MA, Leardini G, Salaffi F, Intorcia M, Bellatreccia A, Dupont D, et al. Assessing the cost-effectiveness of biologic agents for the management of moderate-to-severe rheumatoid arthritis in anti-TNF inadequate responders in Italy: a modelling approach. Clin Exp Rheumatol. 2011;29(4):633–41.
Bansback NJ, Brennan A, Ghatnekar O. Cost effectiveness of adalimumab in the treatment of patients with moderate to severe rheumatoid arthritis in Sweden. Ann Rheum Dis. 2005;64(7):995–1002.
Beresniak A, Ariza-Ariza R, Garcia-Llorente JF, Ramirez-Arellano A, Dupont D. Modelling cost-effectiveness of biologic treatments based on disease activity scores for the management of rheumatoid arthritis in Spain. Int J Inflamm. 2011;2011:727634.
Malottki K, Barton P, Tsourapas A, Uthman AO, Liu Z, Routh K, et al. Adalimumab, etanercept, infliximab, rituximab and abatacept for the treatment of rheumatoid arthritis after the failure of a tumour necrosis factor inhibitor: a systematic review and economic evaluation. Health Technol Assess. 2011;15(14):1–278.
Barton P. Development of the Birmingham Rheumatoid Arthritis Model: past, present and future plans. Rheumatology (Oxford). 2011;50 Suppl 4:iv32–8.
Barton P, Jobanputra P, Wilson J, Bryan S, Burls A. The use of modelling to evaluate new drugs for patients with a chronic condition: the case of antibodies against tumour necrosis factor in rheumatoid arthritis. Health Technol Assess. 2004;8(11):iii, 1–91.
Chen YF, Jobanputra P, Barton P, Jowett S, Bryan S, Clark W, et al. A systematic review of the effectiveness of adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis in adults and an economic evaluation of their cost-effectiveness. Health Technol Assess. 2006;10(42):iii, iv, xi–xiii, 1–229.
Clark W, Jobanputra P, Barton P, Burls A. The clinical and cost-effectiveness of anakinra for the treatment of rheumatoid arthritis in adults: a systematic review and economic analysis. Health Technol Assess. 2004;8(18):iii, iv, ix–x, 1–105.
Jobanputra P, Barton P, Bryan S, Burls A. The effectiveness of infliximab and etanercept for the treatment of rheumatoid arthritis: a systematic review and economic evaluation. Health Technol Assess. 2002;6(21):1–110.
Brennan A, Bansback N, Reynolds A, Conway P. Modelling the cost-effectiveness of etanercept in adults with rheumatoid arthritis in the UK. Rheumatology (Oxford). 2004;43(1):62–72.
Brennan A, Bansback N, Nixon R, Madan J, Harrison M, Watson K, et al. Modelling the cost effectiveness of TNF-alpha antagonists in the management of rheumatoid arthritis: results from the British Society for Rheumatology Biologics Registry. Rheumatology (Oxford). 2007;46(8):1345–54.
Chiou CF, Choi J, Reyes CM. Cost-effectiveness analysis of biological treatments for rheumatoid arthritis. Expert Rev Pharmacoecon Outcomes Res. 2004;4(3):307–15.
Choi HK, Seeger JD, Kuntz KM. A cost effectiveness analysis of treatment options for methotrexate-naive rheumatoid arthritis. J Rheumatol. 2002;29(6):1156–65.
Davies A, Cifaldi MA, Segurado OG, Weisman MH. Cost-effectiveness of sequential therapy with tumor necrosis factor antagonists in early rheumatoid arthritis. J Rheumatol. 2009;36(1):16–26.
Diamantopoulos A, Benucci M, Capri S, Berger W, Wintfeld N, Giuliani G, et al. Economic evaluation of tocilizumab combination in the treatment of moderate-to-severe rheumatoid arthritis in Italy. J Med Econ. 2012;15(3):576–85.
Finckh A, Bansback N, Marra CA, Anis AH, Michaud K, Lubin S, et al. Treatment of very early rheumatoid arthritis with symptomatic therapy, disease-modifying antirheumatic drugs, or biologic agents: a cost-effectiveness analysis. Ann Intern Med. 2009;151(9):612–21.
Hallinen TA, Soini EJ, Eklund K, Puolakka K. Cost–utility of different treatment strategies after the failure of tumour necrosis factor inhibitor in rheumatoid arthritis in the Finnish setting. Rheumatology (Oxford). 2010;49(4):767–77.
Kielhorn A, Porter D, Diamantopoulos A, Lewis G. UK cost–utility analysis of rituximab in patients with rheumatoid arthritis that failed to respond adequately to a biologic disease-modifying antirheumatic drug. Curr Med Res Opin. 2008;24(9):2639–50.
Lekander I, Borgstrom F, Svarvar P, Ljung T, Carli C, van Vollenhoven RF. Cost-effectiveness of real-world infliximab use in patients with rheumatoid arthritis in Sweden. Int J Technol Assess Health Care. 2010;26(1):54–61.
Kobelt G, Jonsson L, Lindgren P, Young A, Eberhardt K. Modeling the progression of rheumatoid arthritis: a two-country model to estimate costs and consequences of rheumatoid arthritis. Arthritis Rheum. 2002;46(9):2310–9.
Kobelt G, Lindgren P, Young A. Modelling the costs and effects of leflunomide in rheumatoid arthritis. Eur J Health Econ. 2002;3(3):180–7.
Kobelt G, Jonsson L, Young A, Eberhardt K. The cost-effectiveness of infliximab (remicade) in the treatment of rheumatoid arthritis in Sweden and the United Kingdom based on the ATTRACT study. Rheumatology (Oxford). 2003;42(2):326–35.
Kobelt G, Lindgren P, Lindroth Y, Jacobson L, Eberhardt K. Modelling the effect of function and disease activity on costs and quality of life in rheumatoid arthritis. Rheumatology (Oxford). 2005;44(9):1169–75.
Kobelt G, Lindgren P, Singh A, Klareskog L. Cost effectiveness of etanercept (Enbrel) in combination with methotrexate in the treatment of active rheumatoid arthritis based on the TEMPO trial. Ann Rheum Dis. 2005;64(8):1174–9.
Kobelt G, Lekander I, Lang A, Raffeiner B, Botsios C, Geborek P. Cost-effectiveness of etanercept treatment in early active rheumatoid arthritis followed by dose adjustment. Int J Technol Assess Health Care. 2011;27(3):193–200.
Lindgren P, Geborek P, Kobelt G. Modeling the cost-effectiveness of treatment of rheumatoid arthritis with rituximab using registry data from southern Sweden. Int J Technol Assess Health Care. 2009;25(2):181–9.
Kobelt G, Lindgren P, Geborek P. Costs and outcomes for patients with rheumatoid arthritis treated with biological drugs in Sweden: a model based on registry data. Scand J Rheumatol. 2009;38(6):409–18.
Maetzel A, Strand V, Tugwell P, Wells G, Bombardier C. Cost effectiveness of adding leflunomide to a 5-year strategy of conventional disease-modifying antirheumatic drugs in patients with rheumatoid arthritis. Arthritis Rheum. 2002;47(6):655–61.
Marra CA, Marion SA, Guh DP, Najafzadeh M, Wolfe F, Esdaile JM, et al. Not all “quality-adjusted life years” are equal. J Clin Epidemiol. 2007;60(6):616–24.
Merkesdal S, Kirchhoff T, Wolka D, Ladinek G, Kielhorn A, Rubbert-Roth A. Cost-effectiveness analysis of rituximab treatment in patients in Germany with rheumatoid arthritis after etanercept-failure. Eur J Health Econ. 2010;11(1):95–104.
Nguyen CM, Bounthavong M, Mendes MA, Christopher ML, Tran JN, Kazerooni R, et al. Cost utility of tumour necrosis factor-alpha inhibitors for rheumatoid arthritis: an application of Bayesian methods for evidence synthesis in a Markov model. Pharmacoeconomics. 2012;30(7):575–93.
Puolakka K, Blafield H, Kauppi M, Luosujarvi R, Peltomaa R, Leikola-Pelho T, et al. Cost-effectiveness modelling of sequential biologic strategies for the treatment of moderate to severe rheumatoid arthritis in Finland. Open Rheumatol J. 2012;6:38–43.
Russell A, Beresniak A, Bessette L, Haraoui B, Rahman P, Thorne C, et al. Cost-effectiveness modeling of abatacept versus other biologic agents in DMARDS and anti-TNF inadequate responders for the management of moderate to severe rheumatoid arthritis. Clin Rheumatol. 2009;28(4):403–12.
Saraux A, Gossec L, Goupille P, Bregman B, Boccard E, Dupont D, et al. Cost-effectiveness modelling of biological treatment sequences in moderate to severe rheumatoid arthritis in France. Rheumatology (Oxford). 2010;49(4):733–40.
Schadlich PK, Zeidler H, Zink A, Gromnica-Ihle E, Schneider M, Straub C, et al. Modelling cost effectiveness and cost utility of sequential DMARD therapy including leflunomide for rheumatoid arthritis in Germany: II. The contribution of leflunomide to efficiency. Pharmacoeconomics. 2005;23(4):395–420.
Soini EJ, Hallinen TA, Puolakka K, Vihervaara V, Kauppi MJ. Cost-effectiveness of adalimumab, etanercept, and tocilizumab as first-line treatments for moderate-to-severe rheumatoid arthritis. J Med Econ. 2012;15(2):340–51.
Spalding JR, Hay J. Cost effectiveness of tumour necrosis factor-alpha inhibitors as first-line agents in rheumatoid arthritis. Pharmacoeconomics. 2006;24(12):1221–32.
Suka M, Yoshida K. Cost effectiveness of leflunomide in the treatment of rheumatoid arthritis in Japan. Expert Rev Pharmacoecon Outcomes Res. 2004;4(6):617–22.
Tanno M, Nakamura I, Ito K, Tanaka H, Ohta H, Kobayashi M, et al. Modeling and cost-effectiveness analysis of etanercept in adults with rheumatoid arthritis in Japan: a preliminary analysis. Mod Rheumatol. 2006;16(2):77–84.
Tosh JC, Wailoo AJ, Scott DL, Deighton CM. Cost-effectiveness of combination nonbiologic disease-modifying antirheumatic drug strategies in patients with early rheumatoid arthritis. J Rheumatol. 2011;38(8):1593–600.
Tosh J, Brennan A, Wailoo A, Bansback N. The Sheffield Rheumatoid Arthritis Health Economic Model. Rheumatology (Oxford). 2011;50 Suppl 4:iv26–31.
Yuan Y, Trivedi D, Maclean R, Rosenblatt L. Indirect cost-effectiveness analyses of abatacept and rituximab in patients with moderate-to-severe rheumatoid arthritis in the United States. J Med Econ. 2010;13(1):33–41.
Vera-Llonch M, Massarotti E, Wolfe F, Shadick N, Westhovens R, Sofrygin O, et al. Cost-effectiveness of abatacept in patients with moderately to severely active rheumatoid arthritis and inadequate response to tumor necrosis factor-alpha antagonists. J Rheumatol. 2008;35(9):1745–53.
Vera-Llonch M, Massarotti E, Wolfe F, Shadick N, Westhovens R, Sofrygin O, et al. Cost-effectiveness of abatacept in patients with moderately to severely active rheumatoid arthritis and inadequate response to methotrexate. Rheumatology (Oxford). 2008;47(4):535–41.
Wailoo AJ, Bansback N, Brennan A, Michaud K, Nixon RM, Wolfe F. Biologic drugs for rheumatoid arthritis in the Medicare program: a cost-effectiveness analysis. Arthritis Rheum. 2008;58(4):939–46.
Welsing PM, Severens JL, Hartman M, van Gestel AM, van Riel PL, Laan RF. The initial validation of a Markov model for the economic evaluation of (new) treatments for rheumatoid arthritis. Pharmacoeconomics. 2006;24(10):1011–20.
Welsing PM, Severens JL, Hartman M, van Riel PL, Laan RF. Modeling the 5-year cost effectiveness of treatment strategies including tumor necrosis factor-blocking agents and leflunomide for treating rheumatoid arthritis in the Netherlands. Arthritis Rheum. 2004;51(6):964–73.
Schipper LG, Kievit W, den Broeder AA, van der Laar MA, Adang EM, Fransen J, et al. Treatment strategies aiming at remission in early rheumatoid arthritis patients: starting with methotrexate monotherapy is cost-effective. Rheumatology (Oxford). 2011;50(7):1320–30.
Barbieri M, Wong JB, Drummond M. The cost effectiveness of infliximab for severe treatment-resistant rheumatoid arthritis in the UK. Pharmacoeconomics. 2005;23(6):607–18.
Wong JB, Singh G, Kavanaugh A. Estimating the cost-effectiveness of 54 weeks of infliximab for rheumatoid arthritis. Am J Med. 2002;113(5):400–8.
Beaudet A, Palmer JL, Timlin L, Wilson B, Bruhn D, Boye KS, et al. Cost–utility of exenatide once weekly compared with insulin glargine in patients with type 2 diabetes in the UK. J Med Econ. 2011;14(3):357–66.
Hiligsmann M, Cooper C, Arden N, Boers M, Branco JC, Luisa Brandi M, et al. Health economics in the field of osteoarthritis: an expert’s consensus paper from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Semin Arthritis Rheum. 2013;43(3):303–13.
The Royal Society. Science as an open enterprise: open data for open science; 2012. http://royalsociety.org/uploadedFiles/Royal_Society_Content/policy/projects/sape/2012-06-20-SAOE.pdf.
Blum S, Vardi M, Brown JB, Russell A, Milman U, Shapira C, et al. Vitamin E reduces cardiovascular disease in individuals with diabetes mellitus and the haptoglobin 2-2 genotype. Pharmacogenomics. 2010;11(5):675–84.
Toumi M, Antonanzas F, Hakkaart L, Lam RW, McCrone P, Persson U, et al. Comprehensive discrete event simulation model for the evaluation of health care technologies in depression. Value Health. 2012;15(7):A282.
Disclosures
The author contributions to this manuscript were as follows:
HvH: study rationale and design, execution of systematic literature search, study selection, execution of stepwise selection, interpretation and reflection, writing of the manuscript.
JS: study rationale and design, study selection, verification of stepwise selection, interpretation and reflection, review of the manuscript, overall guarantor of the study.
ATD: study selection, feedback on study execution and interpretation, review of the manuscript.
AB: feedback on study execution and interpretation, review of the manuscript.
HvH is employed by AstraZeneca and affiliated with the Erasmus University Rotterdam Institute of Health Policy & Management by means of a PhD hospitality agreement. JS’s supervision of HvH is compensated by AstraZeneca on the basis of an unrestricted supervision grant to his institution. In the field of rheumatoid arthritis, JS has received research grants from Pfizer. AB has received research grants from Merck, AbbVie and Amgen. HvH, JS, ATD and AB declare no conflict of interest regarding the topics studied and the results presented in this publication.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
van Haalen, H.G.M., Severens, J.L., Tran-Duy, A. et al. How to Select the Right Cost-Effectiveness Model?. PharmacoEconomics 32, 429–442 (2014). https://doi.org/10.1007/s40273-014-0139-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-014-0139-9