Abstract
Background
Chronic hepatitis C is the leading cause of chronic liver disease, representing a significant burden in terms of morbidity, mortality and costs. A new scenario of therapy for hepatitis C virus (HCV) genotype 1 infection is being established with the approval of two effective HCV protease inhibitors (PIs) in combination with the standard of care (SOC), peginterferon and ribavirin.
Objective
Our objective was to estimate the cost effectiveness of combination therapy with new PIs (boceprevir and telaprevir) plus peginterferon and ribavirin versus SOC in treatment-naive patients with HCV genotype 1 according to data obtained from clinical trials (CTs).
Methods
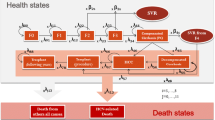
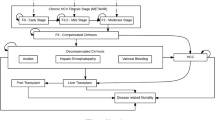
A Markov model simulating chronic HCV progression was used to estimate disease treatment costs and effects over patients’ lifetimes, in the Spanish national public healthcare system. The target population was treatment-naive patients with chronic HCV genotype 1, demographic characteristics for whom were obtained from the published pivotal CTs SPRINT and ADVANCE. Three options were analysed for each PI based on results from the two CTs: universal triple therapy, interleukin (IL)-28B-guided therapy and dual therapy with peginterferon and ribavirin. A univariate sensitivity analysis was performed to evaluate the uncertainty of certain parameters: age at start of treatment, transition probabilities, drug costs, CT efficacy results and a higher hazard ratio for all-cause mortality for patients with chronic HCV. Probabilistic sensitivity analyses were also carried out.
Results
Incremental cost-effectiveness ratios (ICERs) of €2012 per quality-adjusted life-year (QALY) gained were used as outcome measures. According to the base-case analysis, using dual therapy as the comparator, the alternative IL28B-guided therapy presents a more favorable ICER (€18,079/QALY for boceprevir and €25,914/QALY for telaprevir) than the universal triple therapy option (€27,594/QALY for boceprevir and €33,751/QALY for telaprevir), with an ICER clearly below the efficiency threshold for medical interventions in the Spanish setting. Sensitivity analysis showed that age at the beginning of treatment was an important factor that influenced the ICER. A potential reduction in PI costs would also clearly improve the ICER, and transition probabilities influenced the results, but to a lesser extent. Probabilistic sensitivity analyses showed that 95 % of the simulations presented an ICER below €40,000/QALY. Post hoc estimations of sustained virological responses of the IL28B-guided therapeutic option represented a limitation of the study.
Conclusion
The therapeutic options analysed for the base-case cohort can be considered cost-effective interventions for the Spanish healthcare framework. Sensitivity analysis estimated an acceptability threshold of the IL28B-guided strategy of patients younger than 60 years.






Similar content being viewed by others
References
European Association of the Study of the Liver. EASL International Consensus Conference on Hepatitis C, Paris, 26–28 February 1999. Consensus statement. J Hepatol. 1999;30(5):956–61.
European Association of the Study of the Liver. 2011 European Association of the Study of the Liver hepatitis C virus clinical practice guidelines. Liver Int. 2012;32(Suppl 1):2–8.
Alter MJ, Kruszon-Moran D, Nainan OV, et al. Prevalence of hepatitis C virus infection in the USA, 1988–1994. N Engl J Med. 1999;341(8):556–62.
Calleja Panero JL, Martinez Porras JL, Albillos Martinez A. Tratamiento de la hepatitis crónica por virus C. Inf Ter Sist Nac Salud. 2001;25:69–77.
Wasley A, Alter MJ. Epidemiology of hepatitis C: geographic differences and temporal trends. Semin Liver Dis. 2000;20(1):1–16.
Ghany MG, Nelson DR, Strader DB, et al. An update on treatment of genotype 1 chronic hepatitis C virus infection: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011;54(4):1433–44.
Poordad F, McCone J Jr, Bacon BR, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364(13):1195–206.
Jacobson IM, McHutchison JG, Dusheiko G, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364(25):2405–16.
Gold M, Siegel JE, Russell LB, et al. Cost-effectiveness in health and medicine. New York: Oxford University Press; 1996.
Drummond MF, O’Brien B, Stoddart GL, Torrance GW. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 2003.
European Medicines Agency. Victrelis: summary of product characteristics [online]. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR__Product_Information/human/002332/WC500109786.pdf (Accessed 21 Aug 2012).
European Medicines Agency. Incivo: summary of product characteristics [online]. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_Product_Information/human/002313/WC500115529.pdf (Accessed 21 Aug 2012).
Poordad F, Bronowicki JP, Gordon SC, et al. Factors that predict response of patients with hepatitis C virus infection to boceprevir. Gastroenterology. 2012;143(3):608–18.
Jacobson IM, Catlett I, Marcellin P, et al. Telaprevir substantially improved SVR rates across all IL28B genotypes in the ADVANCE trial. J Hepatol. 2011;54:S542–3.
Montes-Cano MA, García-Lozano JR, Abad-Molina C, et al. Interleukin-28B genetic variants and hepatitis virus infection by different viral genotypes. Hepatology. 2010;52(1):33–7.
Available from URL: http://www.portalfarma.com (Accessed 21 Aug 2012).
Buti M, Casado MA, Fosbrook L, et al. Cost effectiveness of the treatment of chronic hepatitis C with interferon-alpha. Gastroenterol Hepatol. 1998;21(4):161–8.
Buti M, Casado MA, Fosbrook L, et al. Financial impact of two different ways of evaluating early virological response to peginterferon-alpha-2b plus ribavirin therapy in treatment-naive patients with chronic hepatitis C virus genotype 1. Pharmacoeconomics. 2005;23(10):1043–55.
San Miguel R, Mar J, Cabasés JM, Guillén-Grima F, Buti M. Cost-effectiveness analysis of therapeutic strategies for patients with chronic hepatitis C previously not responding to interferon. Aliment Pharmacol Ther. 2003;17(6):765–73.
Cacoub P, Bourlière M, Lübbe J, et al. Dermatological side effects of hepatitis C and its treatment: patient management in the era of direct-acting antivirals. J Hepatol. 2012;56(2):455–63.
Hézode C. Boceprevir and telaprevir for the treatment of chronic hepatitis C: safety management in clinical practice. Liver Int. 2012;32(Suppl 1):32–8.
Tungol A, Rademacher K, Schafer JA. Formulary management of the protease inhibitors boceprevir and telaprevir for chronic hepatitis C virus. J Manag Care Pharm. 2011;17(9):685–94.
Grieve R, Roberts J, Wright M, et al. Cost effectiveness of interferon alpha or peginterferon alpha with ribavirin for histologically mild chronic hepatitis C. Gut. 2006;55(9):1332–8.
Sonnenberg FA, Beck R. Markov models in medical decision making: a practical guide. Med Decis Making. 1993;13(4):322–38.
Townsend R, McEwan P, Kim R, et al. Structural frameworks and key model parameters in cost-effectiveness analyses for current and future treatments of chronic hepatitis C. Value Health. 2011;14(8):1068–77.
Briggs A, Sculpher M, Claxton K. Decision modelling for health economic evaluation. Oxford: Oxford University Press; 2006.
Planas R, Ballesté B, Alvarez MA, et al. Natural history of decompensated hepatitis C virus-related cirrhosis. A study of 200 patients. J Hepatol. 2004;40(5):823–30.
Fattovich G, Giustina G, Degos F, et al. Morbidity and mortality in compensated cirrhosis type C: retrospective follow-up study of 384 patients. Gastroenterology. 1997;112(2):463–72.
Organización Nacional de Transplantes. Registro español de transplante hepático. Memoria de resultados; 2010.
Siebert U, Sroczynski G, Rossol S, et al. Cost effectiveness of peginterferon alpha-2b plus ribavirin versus interferon alpha-2b plus ribavirin for initial treatment of chronic hepatitis C. Gut. 2003;52(3):425–32.
Salomon JA, Weinstein MC, Hammitt JK, et al. Empirically calibrated model of hepatitis C virus infection in the United States. Am J Epidemiol. 2002;156:761–73.
Comité de evaluación de nuevos medicamentos de uso Hospitalario. Telaprevir (Incivo®) Nº 20, marzo; 2012. http://www.osakidetza.euskadi.net/r85-pkcevi03/es/contenidos/informacion/cevime_ambito_hospitalario/es_cevime/.
Comité de evaluación de nuevos medicamentos de uso Hospitalario. Boceprevir (Victrelis®) Nº 19, marzo 2012. http://www.osakidetza.euskadi.net/r85-pkcevi03/es/contenidos/informacion/cevime_ambito_hospitalario/es_cevime/.
Liu S, Cipriano LE, Holodniy M, et al. New protease inhibitors for the treatment of chronic hepatitis C: a cost-effectiveness analysis. Ann Intern Med. 2012;156(4):279–90.
Bennett WG, Inoue Y, Beck JR, et al. Estimates of the cost-effectiveness of a single course of interferon-alpha 2b in patients with histologically mild chronic hepatitis C. Ann Intern Med. 1997;127:855–65.
O’Brien BJ, Drummond MF, Labelle RJ, et al. In search of power and significance: issues in the design and analysis of stochastic cost-effectiveness studies in health care. Med Care. 1994;32(2):150–63.
Briggs AH, Goeree R, Blackhouse G, et al. Probabilistic analysis of cost-effectiveness models: choosing between treatment strategies for gastroesophageal reflux disease. Med Decis Making. 2002;22(4):290–308.
Claxton K, Neumann PJ, Araki S, et al. Bayesian value-of-information analysis: an application to a policy model of Alzheimer’s disease. Int J Technol Asses Health Care. 2001;17(1):38–55.
Wright M, Grieve R, Roberts J, et al. on behalf of the UK Mild Hepatitis C Trial Investigators. Health benefits of antiviral therapy for mild chronic hepatitis C: randomised controlled trial and economic evaluation. Health Technol Assess. 2006;10(21):1–113.
Eichler HG, Kong SX, Gerth WC, et al. Use of cost-effectiveness analysis in health-care resource allocation decision-making: how are cost-effectiveness thresholds expected to emerge? Value Health. 2004;7(5):518–28.
Sacristan JA, Oliva J, del Llano J, et al. What is an efficient health technology in Spain? Gac Sanit. 2002;4:334–43.
Criterios y recomendaciones generales para el tratamiento con Boceprevir y Telaprevir de la hepatitis crónica C (VHC) en pacientes monoinfectados. http://www.aemps.gob.es/medicamentosUsoHumano/informesPublicos/home.htm.
Conflict of interest
The authors have no conflicts of interest to declare. This analysis was performed as academic research and was not supported by any pharmaceutical company, government agency or grant. This manuscript represents the personal opinion of the authors and does not necessarily represent the views or policy of the Spanish Agency for Medicines and Health Care Products.
Authors’ contributions
Dr Antonio Blázquez: contributions to the study conception and design, analysis of clinical trials: review and results analysis, Markov model revision, results analysis.
Dr Ramón San Miguel: contributions to the study conception and design, analysis of clinical trials: review and results analysis, Markov model revision, results analysis.
Dr Javier Mar: contributions to the study conception and design, Markov model conception, design and validation, Markov model revision, results analysis.
All the authors have participated in drafting the article and revising it critically for intellectual content, final approval of the version to be published.
Dr Antonio Blázquez is the guarantor for the overall content.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Blázquez-Pérez, A., San Miguel, R. & Mar, J. Cost-Effectiveness Analysis of Triple Therapy with Protease Inhibitors in Treatment-Naive Hepatitis C Patients. PharmacoEconomics 31, 919–931 (2013). https://doi.org/10.1007/s40273-013-0080-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-013-0080-3