Abstract
Background
Cost-effectiveness evidence is increasingly considered in the reimbursement decisions of pharmaceuticals. In some jurisdictions such as the UK and Canada, pharmaceutical manufacturers are required to submit economic evaluations when seeking reimbursement.
Objectives
Our objectives were to describe the role of economic evidence in the cancer drug review process in Canada, and to investigate the nature of problems encountered in the review and interpretation of economic evidence used in the process.
Design
We conducted a retrospective review of cancer drug review meeting minutes and reviewers’ comments on pharmacoeconomic studies submitted to the oncology drug review process in Canada.
Data Sources
We used pharmacoeconomic reviewers’ reports and relevant cancer drug review expert advisory committee meeting minutes during the first year of the review process (April 2007 to March 2008).
Results
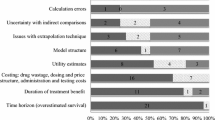
Fifteen economic submissions were reviewed. One-third of the studies had flaws significant enough that the advisory committee could not determine the cost effectiveness of the drugs from the results. The common issues outlined by the reviewers and committee were related to the uncertainty of comparative clinical benefits, quality of life and costs. The reviewers felt that few analyses provided sufficient sensitivity analyses around key variables to assess the robustness of results. Most problems identified by reviewers are simple to fix and do not involve advanced methods.
Conclusions
Canada has a separate review process for making cancer drug funding recommendations, and this process uses both clinical and economic evidence. The committee could not determine the value for money of the drugs from several of the submitted pharmacoeconomic analyses. Transparent analyses and detailed critique of evidence are crucial to the use of economic evidence in reimbursement decisions. Rigorous evaluation is resource intensive and may benefit from a shared drug review process among several jurisdictions.

Similar content being viewed by others
References
Canadian Institute for Health Information. Drug expenditure in Canada 1985 to 2009. Ottawa: CIHI; 2010.
Clement FM, Harris A, Li JJ, Yong K, Lee KM, Manns BJ. Using effectiveness and cost-effectiveness to make drug coverage decisions: a comparison of Britain, Australia, and Canada. JAMA. 2009;302(13):1437–43.
Hill SR, Mitchell AS, Henry DA. Problems with the interpretation of pharmacoeconomic analyses: a review of submissions to the Australian Pharmaceutical Benefits Scheme. JAMA. 2000;283(16):2116–21.
Morgan SG, McMahon M, Mitton C, Roughead E, Kirk R, Kanavos P, et al. Centralized drug review processes in Australia, Canada, New Zealand, and the United Kingdom. Health Aff (Millwood). 2006;25(2):337–47.
Späth HM, Allenet B, Carrère MO. L’utilisation de l’information économique dans le secteur de la santé: le choix des médicaments à inclure dans les livrets thérapeutiques hospitaliers. J Econ Med. 2000;18(3–4):147–61.
Eddama O, Coast J. A systematic review of the use of economic evaluation in local decision-making. Health Policy. 2008;86(2–3):129–41.
Williams I, McIver S, Moore D, Bryan S. The use of economic evaluations in NHS decision-making: a review and empirical investigation. Health Technol Assess 2008; 12(7):iii, ix-iii, 175.
PausJenssen AM, Singer PA, Detsky AS. Ontario’s formulary committee: how recommendations are made. Pharmacoeconomics. 2003;21(4):285–94.
Health Canada. Canada’s health care system (Medicare). Ottawa: Health Canada; 2010. http://www.hc-sc.gc.ca/hcs-sss/medi-assur/index-eng.php. Accessed 16 Apr 2011.
Ontario Ministry of Health and Long-Term Care. Inter-Provincial Joint Oncology Drug Review Process. Toronto: Government of Ontario; 2011. http://www.health.gov.on.ca/english/providers/program/drugs/drug_submissions/inter_oncology_drugs.html. Accessed 16 Apr 2011.
Rocchi A, Miller E, Hopkins RB, Goeree R. Common drug review recommendations: an evidence base for expectations? Pharmacoeconomics. 2012;30(3):229–46.
Chauhan D, Miners AH, Fischer AJ. Exploration of the difference in results of economic submissions to the National Institute of Clinical Excellence by manufacturers and assessment groups. Int J Technol Assess Health Care. 2007;23(1):96–100.
Rennie D, Luft HS. Pharmacoeconomic analyses: making them transparent, making them credible. JAMA. 2000;283(16):2158–60.
Miners AH, Garau M, Fidan D, Fischer AJ. Comparing estimates of cost effectiveness submitted to the National Institute for Clinical Excellence (NICE) by different organisations: retrospective study. BMJ. 2005;330(7482):65.
Friedberg M, Saffran B, Stinson TJ, Nelson W, Bennett CL. Evaluation of conflict of interest in economic analyses of new drugs used in oncology. JAMA. 1999;282(15):1453–7.
Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-effectiveness in health and medicine. New York: Oxford University Press; 1996.
Canadian Agency for Drugs and Technologies in Health. Guidelines for the economic evaluation of health technologies: Canada. 3rd ed. Ottawa: CADTH; 2006.
Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ. 1996;313(7052):275–83.
Ontario Ministry of Health and Long-Term Care. EO decisions and CED recommendations. Toronto: Government of Ontario; 2011. http://www.health.gov.on.ca/english/providers/program/drugs/ced_rec_table.html. Accessed 16 Apr 2011.
Pan-Canadian Oncology Drug Review. Toronto: pCODR; 2011. http://www.pcodr.ca. Accessed 25 Jan 2012.
Acknowledgments
The Pharmacoeconomics Research Unit receives funding from CCO, which receives funding from the Ontario Ministry of Health and Long-Term Care. The views expressed and any omissions in this paper are those of the authors alone.
Author contributions
As the first author, Jean H.E. Yong designed the study, examined and abstracted data from the pharmacoeconomic reviewers’ reports and CED/CCO subcommittee meeting minutes, and analysed them. Jean H.E. Yong also wrote the draft, revised the manuscript and prepared the manuscript for publication. Jaclyn Beca contributed to subsequent revisions of this manuscript. As the senior author, Jeffrey S. Hoch conceived of the research idea, oversaw the design and data collection, reviewed the abstracted data and analysis, and contributed to subsequent revisions of this manuscript. Jean H.E. Yong is the guarantor for the content of this paper.
Conflicts of interest
No funding was received for the study and/or preparation of the paper and the authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yong, J.H.E., Beca, J. & Hoch, J.S. The Evaluation and Use of Economic Evidence to Inform Cancer Drug Reimbursement Decisions in Canada. PharmacoEconomics 31, 229–236 (2013). https://doi.org/10.1007/s40273-012-0022-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-012-0022-5