Abstract
Introduction
Doxapram is used as a third-line treatment for apnea unresponsive to caffeine and continuous positive airway pressure (CPAP) in preterm infants.
Objectives
The objectives of this study were to compare the effects of dosing adjusted for gender and postmenstrual age (PMA) (GrA) versus infants’ weight alone (GrW) on doxapram plasma levels, clinical efficacy, and side effects.
Methods
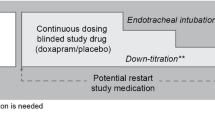
This was a randomized, double-blind study, including premature infants for whom optimized caffeine and CPAP therapy for apnea of prematurity had failed. Failure was defined as the persistence of more than one significant apnea per hour over an 8-h period. Plasma levels of doxapram and ketodoxapram were measured with high-performance liquid chromatography (HPLC) 48 h after the onset of treatment. Dosing aimed to maintain the combined doxapram and ketodoxapram plasma level in the therapeutic range of 1.5–4 mg/l. Infants were followed-up for 4 days after the onset of treatment.
Results
A total of 85 infants were included: 46 in GrW (27.7 ± 1.9 weeks’ gestational age [GA]), 39 in GrA (27.9 ± 1.4 weeks’ GA); available plasma levels showed that 25 of 40 in the GrW group and 27 of 37 in the GrA group had levels within the therapeutic range (p = 0.344). Of note, plasma level variance was significantly higher in GrW for doxapram + ketodoxapram (1.87 vs. 0.89; p = 0.028). Clinical efficacy was better in the GrA group, with a reduction from 32 to 3 of 38 (76 %) infants with significant apnea versus 30 to 5 of 45 (56 %) in the GrW group (p < 0.001). No adverse effects were observed during the study.
Conclusions
Taking gender and PMA into account for doxapram dosing did not significantly increase the number of infants with a plasma level in the therapeutic range. However, it improved plasma level stability and clinical efficacy without adverse effects.
ClinicalTrials.gov number: NCT00389909.


Similar content being viewed by others
References
Bairam A. Low-dose doxapram for apnoea of prematurity. Lancet. 1986;1(8484):793–4.
Peliowski A. A blinded, randomized, placebo-controlled trial to compare theophylline and doxapram for the treatment of apnea of prematurity. J Pediatr. 1990;116:648–53.
Robson RH. A pharmacokinetic study of doxapram in patients and volunteers. Br J Clin Pharmacol. 1979;7:81–7.
Bairam A. Pharmacodynamic effects and pharmacokinetic profiles of keto-doxapram and doxapram in newborn lambs. Pediatr Res. 1990;28:142–6.
Barbé F. Severe side effects and drug plasma concentrations in preterm infants treated with doxapram. Ther Drug Monit. 1999;21:547–52.
Ten Hove CH, Vliegenthart RJ, Te Pas AB, et al. Long-Term Neurodevelopmental outcome after Doxapram for Apnea of Prematurity. Neonatology. 2016;110:21–6.
Caturla L. Sex-and age-related differences in doxapram pharmacokinetics in neonates: a population analysis. Paediatr Perinat Drug Ther. 2004,6:53–54.
Hascoet JM. Risks and benefits of therapies for apnoea in premature infants. Drug Saf. 2000;23:363–79.
Maillard C. QT interval lengthening in premature infants treated with doxapram. Clin Pharmacol Ther. 2001;70:540–5.
Hascoet JM. The safety and efficacy of nitric oxide therapy in premature infants. J Pediatr. 2005;146:318–23.
Ancel PY. Survival and morbidity of preterm children born at 22 through 34 weeks’ gestation in France in 2011: results of the EPIPAGE-2 cohort study. JAMA Pediatr. 2015;169:230–8.
Morley CJ. Nasal CPAP or intubation at birth for very preterm infants. N Engl J Med. 2008;358:700–8.
Tremblay LN. Pathogenesis of ventilator-induced lung injury: trials and tribulations. Am J Physiol Lung Cell Mol Physiol. 2005;288:L596–8.
Jobe AH. Mechanisms initiating lung injury in the preterm. Early Hum Dev. 1998;53:81–94.
Fanni D. Cytochrome P450 genetic polymorphism in neonatal drug metabolism: role and practical consequences towards a new drug culture in neonatology. Int J Immunopathol Pharmacol. 2014;27:5–13.
Hamon I, Valdes V, Franck P, et al. Gender-dependent differences in glutathione (GSH) metabolism in very preterm infants. Arch Pediatr. 2011;18:247–52.
Ogawa Y, Irikura M, Kobaru Y, et al. Population pharmokinetics of doxapram in low-birth-weight Japanese infants with apneas. Eur J Pediatr. 2015;174:509–18.
Henderson-Smart DJ. Doxapram versus methylxanthine for apnea in preterm infants. Cochrane Database Syst Rev. 2000;(4):CD000075.
Henderson-Smart DJ. Doxapram treatment for apnea in preterm infants. Cochrane Database Syst Rev. 2000;(2):CD000074 (Update in: Cochrane Database Syst Rev. 2001;(4):CD000074).
Hayakawa F, Hakamada S, Kuno K, et al. Doxapram in the treatment of idiopathic apnea of prematurity: Desirable dosage and serum concentrations. J Pediatr. 1986;109:138–40.
Peletier LA. Dynamics of target-mediated drug disposition. Eur J Pharm Sci. 2009;38:445–64.
Barrington KJ, Finer NN, Torok-Both G, et al. Dose-response relationship of doxapram in the therapy for refractory idiopathic apnea of prematurity. Pediatrics. 1987;80:22–7.
Bénard M. Determinants of doxapram utilization: a survey of practice in the French Neonatal and Intensive Care Units. Arch Pediatr. 2005;12:151–5.
Eyal F, Alpan G, Sagi E, et al. aminophylline versus doxapram in idiopathic apnea of prematurity: a double-blind controlled study. Pediatrics. 1985;75:709–13.
Tay-Uyboco J. Clinical and physiological responses to prolonged nasogastric administration of doxapram for apnea of prematurity. Biol Neonate. 1991;59:190–200.
Huon C, Rey E, Mussat P, et al. Low-dose doxapram for treatment of apnoea following early weaning in very low birthweight infants: a randomized, double-blind study. Acta Paediatr. 1998;87(11):1180–4.
Acknowledgments
The authors thank Marie-Christine Buchweiller, Sabine Guignon (Research Nurses), and Anne-Fleur André (Research Technician) for data monitoring and management.
Author contributions
Greze E. acquired and interpreted the data, wrote the first draft of the manuscript, critically revised the manuscript, and approved the final submitted version. Hamon I. conceptualized the study, interpreted the data, critically revised the manuscript, and approved the final submitted version. Benard M. acquired the data, critically revised the manuscript, and approved the final submitted version. Casper C. conceptualized the study, acquired the data, critically revised the manuscript, and approved the final submitted version. Haddad F.E. acquired the data, monitored the data collected, critically revised the manuscript, and approved the final submitted version. Boutroy M-J conceptualized and designed the study, critically revised the manuscript, and approved the final submitted version. Hascoët J-M conceptualized the study, analyzed and interpreted the data, critically reviewed and revised the manuscript, and approved the final submitted manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Greze E, Benard M, Hamon I, Casper C, Haddad F.E, Boutroy M-J, Hascoët J-M have no conflicts of interest that are directly related to the content of this study.
Funding
No sources of funding were used to conduct this study or prepare this manuscript.
Ethical approval/informed consent
The study was approved by the Ethical Committee (Number: CPPESTIII-06.03.09). Infants were included in the study after parental consent was obtained.
Rights and permissions
About this article
Cite this article
Greze, E., Benard, M., Hamon, I. et al. Doxapram Dosing for Apnea of Prematurity Based on Postmenstrual Age and Gender: A Randomized Controlled Trial. Pediatr Drugs 18, 443–449 (2016). https://doi.org/10.1007/s40272-016-0192-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-016-0192-2