Abstract
Background
Content valid, patient-reported outcome (PRO) measures of major depressive disorder (MDD) symptoms are needed to assess MDD treatment benefit. While a range of questionnaires are currently available to evaluate aspects of depression from the patient’s perspective, their comprehensiveness and qualitative development histories are unclear.
Objective
The objective of this study was to describe the process and results of the preliminary qualitative development of a new symptom-based PRO measure intended to assess treatment benefit in MDD clinical trials.
Methods
Qualitative interviews were conducted with adult MDD patients in the USA who recently experienced a major depressive episode. Experienced interviewers conducted concept elicitation (CE) and cognitive interviews using semi-structured interview guides. The CE interview guide was used to elicit spontaneous reports of symptom experiences along with probing to further explore and confirm concepts. The cognitive interview guide was developed to evaluate concept relevance, understandability, and structure of the draft items, and to facilitate further instrument refinement.
Results
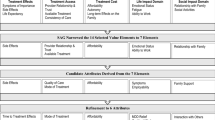
Forty patients participated in the CE interviews. A total of 3022 symptom codes, representing 84 different concepts were derived from the transcripts. Data from the CE interviews were considered alongside existing literature and clinical expert opinion during an item-generation process, leading to development of a preliminary version of the Symptoms of Major Depressive Disorder Scale (SMDDS). Fifteen patients participated in three waves of cognitive interviews, during which the SMDDS was further refined.
Conclusions
The SMDDS is a 35-item PRO measure intended for use as an endpoint in MDD clinical trials to support medical product labeling. The SMDDS uses a 7-day recall period and verbal rating scales. It was developed in accordance with the US Food and Drug Administration (FDA)’s PRO Guidance and best practices. Qualitative interviews have provided evidence for content validity. Future quantitative studies will confirm the SMDDS’s measurement properties and support FDA qualification.



Similar content being viewed by others
References
Kessler RC, Demler O, Frank RG, Olfson M, Pincus HA, Walters EE, et al. Prevalence and treatment of mental disorders, 1990 to 2003. N Engl J Med. 2005;352:2515–23.
Greenberg PE, Fournier AA, Sisitsky T, Pike CT, Kessler RC. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry. 2015;76(2):155–62.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington: American Psychiatric Publishing; 2013.
Hamilton M. A rating scale for depression. J Neurol Neurosur Psychiatry. 1960;23:56–62.
Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9.
Foley DL, Neale MC, Gardner CO, Pickles A, Kendler KS. Major depression and associated impairment: same or different genetic and environmental risk factors? Am J Psychiatry. 2003;160:2128–33.
Uher R, Perlis RH, Placentino A, Dernovšek MA, Henigsberg N, Mors O, et al. Self-report and clinician-rated measures of depression severity: can one replace the other? Depress Anxiety. 2012;29:1043–9.
Coons SJ, Kothari S, Monz BU, Burke LB. The Patient-Reported Outcome (PRO) Consortium: filling measurement gaps for PRO endpoints to support labeling claims. Clin Pharmacol Ther. 2011;90:743–8.
US Food and Drug Administration. Guidance for industry and FDA staff: qualification process for drug development tools. 2014. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM230597.pdf. Accessed 29 Jan 2014.
US Food and Drug Administration. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims. 2009. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM193282.pdf. Accessed 6 Jun 2013.
Ball S, Dedios C, Abraham L. The patient’s perspective on major depressive disorder: what do we know? J Ment Health Policy Econ. 2012;15(Suppl 1):S1.
Blum SI, Bush EN, Bushnell DM. Systematic review of patient-reported outcome measures used to assess symptoms associated with major depressive disorder. J Ment Health Policy. 2012;15:S2–3.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed., text revision. Washington, DC: American Psychiatric Association; 2000.
Friese S. ATLAS.ti 7 User Guide and Reference. Berlin: ATLAS.ti Scientific Software Development GmbH. 2014. http://atlasti.com/wp-content/uploads/2014/05/atlasti_v7_manual_en_201409.pdf. Accessed 10 June 2015.
Patrick DL, Burke LB, Gwaltney CJ, Leidy NK, Martin ML, Molsen E, et al. Content validity—establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO good research practices task force report: part 1—eliciting concepts for a new PRO instrument. Value Health. 2011;14:967–77.
Brod M, Tesler LE, Christensen TL. Qualitative research and content validity: developing best practices based on science and experience. Qual Life Res. 2009;18:1263–78.
Patrick DL, Burke LB, Gwaltney CJ, Leidy NK, Martin ML, Molsen E, et al. Content validity—establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO Good Research Practices Task Force report: part 2—assessing respondent understanding. Value Health. 2011;14:978–88.
Parker G, Roy K, Hadzi-Pavlovic D, Wilhelm K, Mitchell P. The differential impact of age on the phenomenology of melancholia. Psychol Med. 2001;31(7):1231–6.
Morriss R, Leese M, Chatwin J, Baldwin D, THREAD Study Group. Inter-rater reliability of the Hamilton Depression Rating Scale as a diagnostic and outcome measure of depression in primary care. J Affect Disord. 2008;111(2–3):204–13.
Bech P, Fava M, Trivedi MH, Wisniewski SR, Rush AJ. Factor structure and dimensionality of the two depression scales in STAR*D using level 1 datasets. J Affect Disord. 2011;132(3):396–400.
Bagby RM, Ryder AG, Schuller DR, Marshall MB. The Hamilton Depression Rating Scale: has the gold standard become a lead weight? Am J Psychiatry. 2004;161(12):2163–77.
Bech P, Allerup P, Gram LF, Reisby N, Rosenberg R, Jacobsen O, Nagy A. The Hamilton Depression Scale. Evaluation of objectivity using logistic models. Acta Psychiatr Scand. 1981;63(3):290–9.
Bech P, Wilson P, Wessel T, Lunde M, Fava M. A validation analysis of two self-reported HAM-D6 versions. Acta Psychiatr Scand. 2009;119(4):298–303.
Licht RW, Qvitzau S, Allerup P, Bech P. Validation of the Bech-Rafaelsen Melancholia Scale and the Hamilton Depression Scale in patients with major depression; is the total score a valid measure of illness severity? Acta Psychiatr Scand. 2005;111(2):144–9.
Hadzi-Pavlovic D, Hickie I, Brodaty H, Boyce P, Mitchell P, Wilhelm K, et al. Inter-rater reliability of a refined index of melancholia: the CORE system. J Affect Disord. 1993;27(3):155–62.
Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54(5):573–83.
Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive therapy of depression. New York: Guilford; 1979.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3rd ed. Washington, DC: American Psychiatric Association; 1980.
Acknowledgments
This research was funded by the following members of the PRO Consortium: AbbVie, Bristol-Myers Squibb, Eli Lilly and Company, Forest Research Institute, Janssen, Pfizer, Roche Products Limited, Shire, Sunovion, and Takeda. The PRO Consortium receives support through Grant U01FD003865 from the United States Food and Drug Administration to the Critical Path Institute.
The authors would like to thank Linda Carpenter, MD and Madhukar Trivedi, MD for providing their clinical experience and insight to the development process as members of the clinical expert panel (in addition to Dr. Michael E. Thase, who also participated as an author). We would also like to thank Carla Ascoytia, Cecilia Dedios, and Matthew Wolfe for their valuable contributions to the study’s data collection and analysis efforts.
Conflict of interest disclosures
Linda S. Deal (Shire: employee, travel support, holder of stock/stock options), Lucy Abraham (Pfizer: employee, travel support, holder of stock/stock options), Steven I. Blum (Actavis/Forest Research Institute: employee, travel support, and stock/stock options), and Elizabeth N. Bush (Eli Lilly: employee, travel support, holder of stock/stock options) disclose their employment and other financial relationships with their respective firms.
Michael E. Thase has provided scientific consultation to Alkermes, AstraZeneca, Bristol-Myers Squibb Company, Dey Pharma, L.P., Eli Lilly and Company, Forest Pharmaceuticals, Inc., Gerson Lehman Group, GlaxoSmithKline, Guidepoint Global, H. Lundbeck A/S, Janssen Pharmaceuticals; MedAvante, Inc., Merck and Co. Inc., Neuronetics, Inc., Novartis, Otsuka, Ortho-McNeil Pharmaceuticals, PamLab, L.L.C., Pfizer (formerly Wyeth-Ayerst Laboratories), Schering-Plough (formerly Organon, Inc.), Shire US Inc., Sunovion Pharmaceuticals, Inc., Takeda (Lundbeck), and Transcept Pharmaceuticals. Dr. Thase receives grant funding from the Agency for Healthcare Research and Quality, Eli Lilly and Company, the National Institute of Mental Health, Otsuka Pharmaceuticals, and Sepracor, Inc. He has equity holdings in MedAvante, Inc. and receives royalty income from the American Psychiatric Foundation, Inc., Guilford Publications, Herald House, Oxford University Press, and W.W. Norton & Company. His wife is employed as the Group Scientific Director for Embryon—formerly Advogent, which does business with BMS and Pfizer/Wyeth.
Kelly P. McCarrier, Mona L. Martin, and Stephen Joel Coons declare no conflicts of interest.
Author contributions
All authors were involved in the conception and planning of the work that led to the manuscript, as well as interpretation of the data. Kelly P. McCarrier and Mona L. Martin also led the collection and analyses of the study data. All authors participated in drafting or critical revisions of the manuscript and approval of the final submitted version. Kelly P. McCarrier acts as overall guarantor for this study.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Steven I. Blum was affiliated with Forest Research Institute, Jersey City, NJ, USA (now part of Actavis) at the time of his involvement in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
McCarrier, K.P., Deal, L.S., Abraham, L. et al. Patient-Centered Research to Support the Development of the Symptoms of Major Depressive Disorder Scale (SMDDS): Initial Qualitative Research. Patient 9, 117–134 (2016). https://doi.org/10.1007/s40271-015-0132-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40271-015-0132-1