Abstract
Introduction
A subcategory of nonalcoholic fatty liver disease (NAFLD), nonalcoholic steatohepatitis (NASH) is characterized by accumulation of fat accompanied by inflammatory infiltration and hepatocellular damage. The active complex of milk thistle is a lipophilic extract from its seeds, comprising three isomers, collectively known as silymarin. Silymarin has demonstrated antioxidant, anti-inflammatory, and antifibrotic properties, and has been extensively studied in the treatment of liver diseases. The majority of published clinical research on silymarin has used Legalon® (Rottapharm/Madaus), containing the patented extract of milk thistle ETHIS-094™ (Euromed). The current study was undertaken to examine the effects of ETHIS-094™ in the Stelic Animal Model (STAM™), a validated and widely used animal model for NASH.
Methods
After 4 h fasting from 4 to 8 weeks of age, 15 male mice in whom NASH had been induced were orally administered, once daily, either (1) vehicle (saline) at a volume of 10 mL/kg, (2) vehicle supplemented with milk thistle at a dose of 500 mg/kg, or (3) vehicle supplemented with milk thistle at a dose of 1,000 mg/kg.
Results
Mean liver weight and the liver-to-body weight ratio were significantly (P < 0.01) decreased in the milk thistle high-dose group compared with the vehicle group. NAFLD activity score (NAS) tended to decrease in the milk thistle treatment groups compared with vehicle group, as did steatosis scores.
Conclusion
Milk thistle extract administration induced a decreasing trend in NAS compared with the vehicle group. Milk thistle induced a numerical decrease of the steatosis score compared with vehicle, and this was accompanied by a statistically significant decrease in liver weight and the liver-to-body weight ratio, implying a potential anti-steatosis effect of milk thistle.
Similar content being viewed by others
1 Introduction
1.1 Background on Nonalcoholic Fatty Liver Disease (NAFLD) and Nonalcoholic Steatohepatitis (NASH)
Nonalcoholic fatty liver disease (NAFLD) is among the most common causes of chronic liver disorders in the Western world [1]. According to the latest practice guidelines [2], NAFLD encompasses the entire spectrum of fatty liver disease in individuals without significant alcohol consumption, ranging from fatty liver to steatohepatitis and cirrhosis. NAFLD is histologically further categorized into nonalcoholic fatty liver (NAFL) and nonalcoholic steatohepatitis (NASH). NAFL is defined as the presence of hepatic steatosis with no evidence of hepatocellular injury in the form of ballooning of the hepatocytes, or no evidence of fibrosis. NASH is characterized by accumulation of fat accompanied by inflammatory infiltration and hepatocellular damage. NASH can progress to cirrhosis, liver failure, and, rarely, liver cancer [2].
The mechanism of the occurrence and progression of the underlying steatosis to liver disease, including the progression to NASH, is not well understood, but is likely driven by several factors involving genetic predisposition [3]. Accordingly, weight reduction, regular physical activity, and insulin-sensitizing drugs have been extensively used, and examined in several studies of NAFLD. Other treatment approaches include special diets, antioxidants, and cytoprotective therapy [3]. In the 1990s, the incidence of NAFLD and NASH began to increase in both adults and children, in parallel with the rise in obesity rates, and are now widely recognized pathological conditions [4]. The estimated prevalence rates of NAFLD and NASH are 20–30 and 3.5–5 %, respectively [5]. Approximately 10–15 % of patients with histologically proven NASH progress to cirrhosis and its sequelae such as liver failure and hepatocellular carcinoma (HCC) [6]. Patients with NASH, moreover, have reduced survival rates [6]. As the prevalence of NASH threatens to increase further, many medications are being evaluated, targeting different steps in the development of hepatic steatosis or its progression to steatohepatitis [4].
1.2 Background on Milk Thistle (MT)
Silybum marianum, commonly known as milk thistle (MT) (family Asteraceae/Compositae), is one of the oldest and most extensively studied plants for the treatment of liver diseases [3]. The active complex of MT is a lipophilic extract from its seeds, comprising three flavonolignan isomers, known collectively as silymarin [3]. Over the years, the efficacy of silymarin has been extensively assessed in chronic models of hepatic damage (e.g., exposure to CCl4, a well-known hepatotoxic agent) [7, 8]. The initial structural and metabolic damage, resulting in hepatocyte cytotoxicity, is associated with abundant generation of reactive oxygen species (ROS), which induce lipoperoxidation of cell membranes and an increased synthesis of cytokines, the latter being directly responsible for the subsequent inflammatory reactions (the marker of which is the typical activation of NF-κB). The activation of Kupffer cells induces proliferation of hepatic stellate cells (HSC), which are known to be responsible for the deposition of collagen-rich extracellular matrix, a process which leads to progressive liver fibrosis [9]. Quite interestingly—even in the absence of any exogenous hepatotoxic challenge—metabolic conditions such as insulin resistance or diabetes have been shown to determine intrahepatic ROS generation and consequent onset of NASH [10]. Therefore, ROS production, inflammation and fibrogenesis represent the most important features of NASH, and are suitable pharmacological targets to explore the efficacy of potential treatments.
Silymarin has been shown to exert (1) antioxidant activity (silymarin is an ROS scavenger and also reduces the loss of endogenous antioxidant enzymes such as glutathione reductase and peroxidase, catalase and superoxide dismutase [7, 11]); (2) anti-inflammatory activity (silymarin interferes with the NF-κB-induced inflammatory cascade, namely, on the leukotrienes release by Kupffer cells [12]); and (3) antifibrotic activity (silymarin has been shown to reduce liver collagen deposition in vivo in models of chronic toxic liver damage [8] and in a primate model of chronic alcoholic liver damage [13]). The same antifibrotic activity has also been recently demonstrated in a dietary model of NASH [14].
Regarding the different silymarin regimens which may be used, it is important to note that variations in the content, dissolution, and oral bioavailability of silibinin (one of the MT extract isomers) between commercially available silymarin-containing products are significant, despite the same declaration of content. Comparisons between studies should therefore be made cautiously, based on the analytical method used [thin-layer chromatography (TLC) vs. high-performance liquid chromatography (HPLC)] and whether free, conjugated, or total silibinin is being measured [3].
1.3 Clinical Applications of MT
Legalon® (Rottapharm/Madaus)Footnote 1 contains a unique extract of MT, ETHIS-094™ (Euromed), developed in 1968 by the German herbal medicine manufacturer Madaus GmbH, now incorporated as the Rottapharm/Madaus group, headquartered in Rottapharm S.p.A, Monza (Italy). It is a benchmark product, and the majority of published clinical research on silymarin has used Legalon®. In addition, the National Institutes of Health (NIH) and National Center for Complementary and Alternative Medicine (NCCAM) have selected Legalon® capsules for their silymarin trials [15].
Clinical trials have reported that Legalon® is effective in both alcoholic steatohepatitis (ASH) [16–23] and NASH [24].
The current study was undertaken to examine the effects of Euromed’s MT extract (ETHIS-094™) in the Stelic Animal Model (STAM™), a validated animal model for metabolically induced NASH. Because of its antioxidant, anti-inflammatory, and antifibrotic activity, discussed above [7, 8, 11–14], we believed that MT would prove beneficial in treating NASH.
2 Materials and Methods
During this study, all institutional and national guidelines for the care and use of laboratory animals were followed.
2.1 Test Substance
MT was provided by Euromed (the sole supplier of the MT extract used in Legalon®) and stored at room temperature. The test substance was weighed into a plastic tube and saline added. The solution was prepared just before administration.
2.2 Induction of NASH
NASH was induced in 15 male mice according to the method used by Fujii et al. [25], consisting of a single subcutaneous injection of 200 μg streptozotocin 2 days after birth (1st hit) and feeding with high-fat diet (HFD; 57 kcal % fat, cat#: HFD32, CLEA Japan, Japan) starting at 4 weeks of age (2nd hit). In this experimental model, fatty liver and the main histological features of NASH are evident by 5 and 7 weeks after birth, respectively.
2.3 Route of Drug Administration
MT was administered by oral route in a volume of 10 mL/kg.
2.4 Treatment Dose
MT was given at doses of 500 and 1,000 mg/kg once daily after 4 h fasting.
2.5 Animals
C57BL/6 mice (15-day pregnant females) were obtained from Charles River Laboratories Japan (Kanagawa, Japan). All animals used in the study were housed and cared for in accordance with the Japanese Pharmacological Society Guidelines for Animal Use.
2.6 Environment
The animals were maintained in a specific pathogen-free facility under controlled conditions of temperature (23 ± 2 °C), humidity (45 ± 10 %), lighting (12 h artificial light and dark cycles; light from 8:00 to 20:00) and air exchange. A high pressure (20 ± 4 Pa) was maintained in the experimental room to prevent contamination of the facility.
2.7 Animal Husbandry
The animals were housed in polycarbonate cages KN-600 (Natsume Seisakusho, Japan) with a maximum of four mice per cage. Sterilized PULMASμ (Material Research Center, Japan) was used for bedding, and replaced once a week.
2.8 Food and Drink
Sterilized solid HFD was provided ad libitum, being placed in the metal lid on top of the cage. Pure water was provided ad libitum from a water bottle equipped with a rubber stopper and a sipper tube. Water bottles were replaced once a week, cleaned and sterilized in an autoclave and reused.
2.9 Animal and Cage Identification
Mice were identified by numbers engraved on earrings. Each cage was labeled with a specific identification code.
2.10 Plasma Biochemistry
For plasma biochemistry, blood samples were collected in polypropylene tubes with anticoagulant (Novo-Heparin, Mochida Pharmaceutical, Japan) and centrifuged at 1,000×g for 15 min at 4 °C. The supernatant was collected and stored at −80 °C until use. The plasma alanine aminotransferase (ALT) levels were measured by FUJI DRY CHEM 7000 (Fuji Film, Japan).
2.11 Histopathological Analyses
For hematoxylin and eosin (HE) staining, sections were cut from paraffin blocks of liver tissue prefixed in Bouin’s solution and stained with Lillie-Mayer’s Hematoxylin (Muto Pure Chemicals, Japan) and eosin solution (Wako Pure Chemical Industries). NAFLD activity score (NAS) was calculated according to the criteria of Kleiner et al. [26].
2.12 Statistical Tests
Statistical analyses were performed using Bonferroni Multiple Comparison Test on GraphPad Prism 4 (GraphPad Software, USA). P values of <0.05 were considered statistically significant. A trend or tendency was assumed when a one-tailed t test returned P values of <0.10. Results were expressed as mean ± SD.
3 Experimental Design and Treatment
3.1 Study Groups
3.1.1 Group 1: Vehicle
Five NASH mice were orally administered vehicle (saline) at a volume of 10 mL/kg once daily after 4 h fasting from 4 to 8 weeks of age.
3.1.2 Group 2: MT Low Dose
Five NASH mice were orally administered vehicle supplemented with MT at a dose of 500 mg/kg once daily after 4 h fasting from 4 to 8 weeks of age.
3.1.3 Group 3: MT High Dose
Five NASH mice were orally administered vehicle supplemented with MT at a dose of 1,000 mg/kg once daily after 4 h fasting from 4 to 8 weeks of age.
3.2 Animal Monitoring and Sacrifice
The viability, clinical signs and behavior of the mice were monitored daily. Body weight was recorded before the treatment. Mice were observed for significant clinical signs of toxicity, moribundity and mortality approximately 60 min after each administration. The animals were sacrificed by exsanguination through direct cardiac puncture under ether anesthesia (Wako Pure Chemical Industries).
4 Results
4.1 Body Weight Changes and General Condition
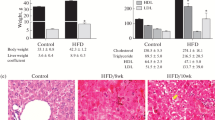
Body weight gradually increased during the treatment period in all groups (Figs. 1, 2).
There were no significant differences in mean body weight between the vehicle group and either the MT low-dose group or the MT high-dose group.
In the present study, none of the animals showed deterioration in general condition.
4.2 Body Weight at the Day of Sacrifice
There were no significant differences in mean body weight between the vehicle group and either the MT low-dose group or the MT high-dose group (vehicle 19.9 ± 1.0 g, MT low dose 20.3 ± 0.9 g, MT high dose 20.3 ± 1.1 g).
4.3 Liver Weight and Liver-to-Body Weight Ratio
Mean liver weight significantly (P < 0.05) decreased in the MT high-dose group compared with the vehicle group (Fig. 3).
The liver weight tended to decrease in the MT low-dose group compared with the vehicle group (vehicle 1,598 ± 89 mg, MT low dose 1,485 ± 96 mg, MT high dose 1,406 ± 93 mg).
The liver-to-body weight ratio was significantly (P < 0.01) decreased in the MT high-dose group compared with the vehicle group (Fig. 4).
The liver-to-body weight ratio tended to decrease in the MT low-dose group compared with the vehicle group (vehicle 8.1 ± 0.7 %, MT low dose 7.3 ± 0.4 %, MT high dose 6.9 ± 0.3 %).
4.4 Plasma Biochemistry
There were no significant differences in plasma ALT levels between the vehicle group and either the MT low-dose group or the MT high-dose group (vehicle 39 ± 12 U/L, MT low dose 48 ± 8 U/L, MT high dose 48 ± 10 U/L).
4.5 Histological Analyses
4.5.1 Hematoxylin and Eosin (HE) Staining and NAFLD Activity Score
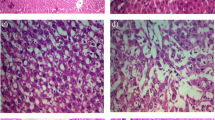
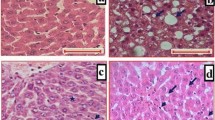
Liver sections from the vehicle group exhibited severe micro- and macrovesicular fat deposition, hepatocellular ballooning and inflammatory cell infiltration (Fig. 5).
The MT treatment groups showed a moderate decrease in fat deposition compared with the vehicle group.
NAS tended to decrease in the MT treatment groups compared with the vehicle group (vehicle 5.4 ± 0.5, MT low dose 4.8 ± 0.4, MT high dose 4.8 ± 0.4), as did steatosis scores, which nearly halved (vehicle 1.4 ± 0.5, MT low dose 0.8 ± 0.4, MT high dose 0.8 ± 0.4) (Table 1; Figs. 6, 7).
5 Discussion
5.1 Summary of Key Methods and Results of this Trial
The present study was aimed at assessing the effect of the MT extract ETHIS-094™ using a validated model of NASH.
This experimental model offered the following advantages: in addition to STAM™ mice showing high practical utility such as uniformity, reproducibility and short turnaround time, this model recapitulates the full spectrum of NAFLD, from simple steatosis to eventual HCC—the worst scenario in human NASH. The histological phenotypes in these mice are also similar to those seen in clinical samples, and the same scoring system used in clinical studies (i.e., NAS) is applicable to this model [27–29]. The applicability of the NAS is one the features that demonstrates the translatability of the STAM™ model. In addition to those three clinical trials, the NAS generated in this model has been published in previous animal studies [25, 30]. It should be noted that this model was used in NASH without measuring glucose metabolism (the rats were diabetic) and was not designed to address the effects of treatment on hepatic carcinogenesis (this can be explained by the relatively short duration of the study—only up to 8 weeks after birth).
In the absence of any relevant data on blood levels of the test substance and previous experience with similar in vivo models, 1,000 mg/kg was selected to be the maximum feasible dosage for repeated oral dosing in such young mice, and was reduced by 50 %, for the purposes of this trial.
The MT extract used in the present study induced a decreasing trend in NAS compared with the vehicle group. Among the three items of the NAS, MT decreased the steatosis score compared with the vehicle group. The lack of power resulting from the small sample size was the most likely reason that this comparison failed to detect a statistical difference between treatments.
The reduction in steatosis score was accompanied by a statistically significant decrease in liver weight and the liver-to-body weight ratio, which implies a potential anti-steatosis effect of MT. The decrease in steatosis correlated well with reduced liver weights, indicating less accumulation of fat in the hepatocytes of animals treated with MT compared with controls. The lack of improvement in hepatocellular ballooning and inflammation should be explored in future studies of longer duration, possibly also in combination with anti-inflammatory/fibrotic agents that lack an anti-steatosis effect.
5.2 Management of NAFLD
Although many pharmacological approaches have been tested [31], the currently recommended therapeutic approach to NAFLD is primarily based on lifestyle intervention [2], with no consensus regarding the ideal pharmacological treatment or a possible nutritional supplement approach. The agents that are used to treat NAFLD should modulate the progression of liver steatosis to inflammation and fibrosis by inhibiting oxidative stress. A multifaceted approach to treating NAFLD, containing several options, is likely to be developed soon. Among those strategic options, the use of nutritional supplements, such as natural antioxidants and hepatoprotective plant products, has been widely accepted in the past decade. One of the best examples of a nutritional supplement that successfully arose from traditional healing practices, silymarin is favored by many clinicians for treating various liver diseases because of its oral efficacy, excellent safety profile, and, most importantly, affordability [3].
5.3 Preclinical Studies of Silymarin
Several pharmacological studies have involved the active components of MT, silymarin, and silibinin, all of which have hepatoprotective, antioxidant, anti-inflammatory, and antifibrotic properties [3]. In particular, in patients with NAFLD, the reduction in insulin resistance, the reduction in lipid peroxidation, and the restoration of glutathione levels obtained with silibinin in diabetic patients, might explain its efficacy in metabolically related NASH [32–34]. On the other hand, both in vitro and in vivo studies have confirmed that silibinin and silymarin reduce HSC activation and proliferation [14, 35].
5.4 Effects of Silymarin on Serum Lipids: Clinical Implications
Sajedianfard et al. [36] studied the effects of a hydroalcoholic extract of silymarin on serum lipid profiles in a streptozotocin-induced diabetic rat model. At the end of 14 days, total cholesterol, triglyceride, LDL-cholesterol and VLDL-cholesterol levels all experienced a dose-dependent reduction, while HDL-cholesterol increased in a dose-dependent manner (P < 0.05). Similarly, Metwally et al. [37] studied the effects of silymarin in a rat model of hypercholesterolemia, and also reported significant (P < 0.01) decreases in serum triglycerides, total cholesterol, LDL-C, and VLDL-C in silymarin-treated animals fed a hypercholesterolemic diet, compared with untreated animals on the same diet.
Through these mechanisms, therefore, it is plausible that silymarin may have a therapeutic role in controlling the onset and/or progression of NASH in patients with metabolic syndrome, particularly in those with insulin resistance.
Regarding the limitations of this study, the small sample size noted above may have contributed to the lack of statistical power of some of the analyses. For example, increasing the sample size would have improved the chances of obtaining a statistically significant difference in the steatosis scores. The sample size was adequate, however, for this pilot study, designed to preliminarily evaluate the potential of candidate compounds by the most robust and reliable analysis—histology. The effects of MT on other parameters such as intrahepatic triglycerides, oxidative stress markers, or antioxidant function markers will be explored in a follow-up to this pilot study for proof of concept. As also noted above, the animal study model was used in NASH without measuring glucose metabolism, and was not designed to address the effects of treatment on hepatic carcinogenesis.
6 Conclusions
In the present study, MT extract (Euromed’s ETHIS-094™) induced a decreasing trend in NAS compared with the vehicle group. Among the three features of NASH, MT induced a numerical decrease in the steatosis score compared with the vehicle group, which was accompanied by a statistically significant decrease in liver weight and the liver-to-body weight ratio, which implies a potential anti-steatosis effect of MT. Legalon® contains the specific extract of S. marianum (ETHIS-094™) that was utilized in this trial, has been evaluated in numerous clinical trials, and is the benchmark silymarin product worldwide.
Notes
Legalon is a registered trademark of Rottapharm/Madaus.
References
Lazo M, Hernaez R, Eberhardt MS, et al. Prevalence of nonalcoholic fatty liver disease in the United States. The Third National Health and Nutrition Examination Survey, 1988–1994. Am J Epidemiol. 2013;178:38–45.
Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–23.
Abenavoli L, Aviello G, Capasso R, Milic N, Capasso F. Milk thistle for treatment of nonalcoholic fatty liver disease. Hepat Mon. 2011;11:173–7.
Pasumarthy L, Srour J. Nonalcoholic steatohepatitis: a review of the literature and updates in management. South Med J. 2010;103:547–50.
Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. AM J Gastroenterol. 2003;98:960–7.
Ekstedt M, Franzén LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–73.
Burczynski FJ, Wang G, Nguyen D, Chen Y, Smith HJ, Gong Y. Silymarin and hepatoprotection. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2012;37:6–10.
Li CC, Hsiang CY, Wu SL, Ho TY. Identification of novel mechanisms of silymarin on the carbon tetrachloride-induced liver fibrosis in mice by nuclear factor-κB bioluminescent imaging-guided transcriptomic analysis. Food Chem Toxicol. 2012;50:1568–75.
George J, Pera N, Phung N, Leclercq I, Yun Hou J, Farrell G. Lipid peroxidation, stellate cell activation and hepatic fibrogenesis in a rat model of chronic steatohepatitis. J Hepatol. 2003;39:756–64.
Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519–23.
Kwon DY, Ahn CW, Choi YJ, Kim YC. Induction of hepatic glutathione synthesis via alterations in sulfur amino acid metabolism in mice treated with silymarin acutely. FASEB J. 2013;27(805):1.
Dehmlow C, Erhard J, de Groot H. Inhibition of Kupffer cell functions as an explanation for the hepatoprotective properties of silibinin. Hepatology. 1996;23:749–54.
Lieber CS, Leo MA, Cao Q, Ren C, DeCarli LM. Silymarin retards the progression of alcohol-induced hepatic fibrosis in baboons. J Clin Gastroenterol. 2003;37:336–9.
Kim M, Yang S-G, Kim JM, Lee J-W, Kim YS, Lee JI. Silymarin suppresses hepatic stellate cell activation in a dietary rat model of non-alcoholic steatohepatitis: analysis of isolated hepatic stellate cells. Int J Mol Med. 2012;30:473–9.
Reddy KR, Belle SH, Fried MW, Afdhal N, Navarro VJ, Hawke RL. Rationale, challenges, and participants in a phase II trial of a botanical product for chronic hepatitis C. Clin Trials. 2012;9:102–12.
Salmi HA, Sarna S. Effect of silymarin on chemical, functional, and morphological alterations of the liver. A double-blind controlled study. Scand J Gastroenterol. 1982;17:517–21.
Ferenci P, Dragosics B, Dittrich H, et al. Randomized controlled trial of silymarin treatment in patients with cirrhosis of the liver. J Hepatol. 1989;9:105–13.
Feher J, Deak G, Muzes G, et al. Hepatoprotective activity of silymarin (Legalon) therapy in patients with chronic liver disease. Orv Hetil. 1989;130:2723–7.
Müzes G, Deák G, Láng I, Nékám K, Niederland V, Fehér J. Effect of silimarin (Legalon) therapy on the antioxidant defense mechanism and lipid peroxidation in alcoholic liver disease (double blind protocol). Orv Hetil. 1990;22;131:863–6. Hungarian.
Velussi M, Cernigoi AM, De Monte A, Dapas F, Caffau C, Zilli M. Long-term (12 months) treatment with an anti-oxidant drug (silymarin) is effective on hyperinsulinemia, exogenous insulin need and malondialdehyde levels in cirrhotic diabetic patients. J Hepatol. 1997;26:871–9.
Saller R, Meier R, Brignoli R. The use of silymarin in the treatment of liver diseases. Drugs. 2001;61:2035–63.
Lucena MI, Andrade RJ, de la Cruz JP, Rodriguez-Mendizabal M, Blanco E, Sánchez-de la Cuesta F. Effects of silymarin MZ-80 on oxidative stress in patients with alcoholic cirrhosis. Results of a randomized, double-blind, placebo-controlled clinical study. Int J Clin Pharmacol Ther. 2002;40:2–8.
Lirussi F, Okolicsanyi L. Cytoprotection in the nineties: experience with ursodeoxycholic acid and silymarin in chronic liver disease. Acta Physiol Hung. 1992;80:363–7.
Buturova LI, Tsybizova TA, Kalinin AV. Use of Legalon in non-alcoholic fatty liver disease [in Russian]. Eksp Klin Gastroenterol. 2010;5:69–75.
Fujii M, Shibazaki Y, Wakamatsu K, et al. A murine model for non-alcoholic steatohepatitis showing evidence of association between diabetes and hepatocellular carcinoma. Med Mol Morphol. 2013;46:141–52.
Kleiner DE, Brunt EM, Van Natta M, Nonalcoholic Steatohepatitis Clinical Research Network, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21.
Klein T, Fujii M, Sandel J, et al. Linagliptin alleviates hepatic steatosis and inflammation in a mouse model of non-alcoholic steatohepatitis. Med Mol Morphol. 2013 [Epub ahead of print] PubMed PMID: 24048504.
Traber PG, Zomer E. Therapy of experimental NASH and fibrosis with galectin inhibitors. PLoS One. 2013;8:e83481.
Kawai D, Takaki A, Nakatsuka A, et al. Hydrogen-rich water prevents progression of nonalcoholic steatohepatitis and accompanying hepatocarcinogenesis in mice. Hepatology. 2012;56:912–21.
Cynis H, Kehlen A, Haegele M, et al. Inhibition of glutaminyl cyclases alleviates CCL2-mediated inflammation of non-alcoholic fatty liver disease in mice. Int J Exp Pathol. 2013;94:217–25.
Nobili V, Svegliati-Baroni G, Alisi A, Miele L, Valenti L, Vajro P. A 360-degree overview of paediatric NAFLD: recent insights. J Hepatol. 2013;58:1218–29.
Huseini HF, Larijani B, Heshmat R, et al. The efficacy of Silybum marianum (L.) Gaertn. (silymarin) in the treatment of type II diabetes: a randomized, double-blind, placebo-controlled, clinical trial. Phytother Res. 2006;20:1036–9.
Hussain SA. Silymarin as an adjunct to glibenclamide therapy improves long-term and postprandial glycemic control and body mass index in type 2 diabetes. J Med Food. 2007;10:543–7.
Yao J, Zhi M, Gao X, Hu P, Li C, Yang X. Effect and the probable mechanisms of silibinin in regulating insulin resistance in the liver of rats with non-alcoholic fatty liver. Braz J Med Biol Res. 2013;46:270–7.
Trappoliere M, Caligiuri A, Schmid M, et al. Silybin, a component of silymarin, exerts anti-inflammatory and anti-fibrogenic effects on human hepatic stellate cells. J Hepatol. 2009;50:1102–11.
Sajedianfard J, Behroozi Z, Nazifi S. The effects of a hydroalcoholic extract of silymarin on serum lipids profiles in streptozotocin induced diabetic rats. Comp Clin Pathol. 2014;23:779–84.
Metwally MAA, El-Gellal AM, El-Sawaisi SM. Effects of silymarin on lipid metabolism in rats. World Appl Sci J. 2009;6:1634–7.
Acknowledgments
During this study, all institutional and national guidelines for the care and use of laboratory animals were followed.
This study was sponsored by Euromed, Barcelona, Spain.
All named authors meet the ICMJE criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
The authors wish to thank Stelic Institute & Co., Tokyo, Japan, for their contributions to the design and conduct of this study, data analyses and data reporting; and Aesculapius Consulting, Inc. for editorial assistance in the preparation of this manuscript. Support for this assistance was provided by Euromed.
Ethical standards
The manuscript does not contain clinical studies or patient data.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Pais, P., D’Amato, M. In Vivo Efficacy Study of Milk Thistle Extract (ETHIS-094™) in STAM™ Model of Nonalcoholic Steatohepatitis. Drugs R D 14, 291–299 (2014). https://doi.org/10.1007/s40268-014-0068-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40268-014-0068-2



 ), low-dose milk thistle (
), low-dose milk thistle ( ), or high-dose milk thistle (
), or high-dose milk thistle ( )
)




