Abstract
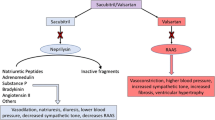
Orally administered sacubitril/valsartan (Entresto®) comprises the neprilysin inhibitor prodrug sacubitril and the angiotensin receptor blocker valsartan, and was recently approved in the USA and the EU for the treatment of chronic heart failure (HF) with reduced ejection fraction (EF). In the PARADIGM-HF trial, the risk of death from cardiovascular causes or first hospitalization for worsening HF (primary composite endpoint) was significantly reduced with sacubitril/valsartan relative to the ACE inhibitor enalapril in patients with chronic HF with reduced EF. Sacubitril/valsartan was generally well tolerated, with a very low risk of angioedema. Therefore, as a first-in-class angiotensin receptor-neprilysin inhibitor, sacubitril/valsartan represents a novel approach to the treatment of chronic HF with reduced EF.
Similar content being viewed by others
References
McMurray JJ, Adamopoulos S, Anker SD, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012. Eur Heart J. 2012;33(14):1787–847.
Hsiao R, Greenberg B. Neprilysin inhibition as a PARADIGM shift in heart failure therapy. Curr Heart Fail Rep. 2016;13(4):172–80.
Jhund PS, McMurray JJV. The neprilysin pathway in heart failure: a review and guide on the use of sacubitril/valsartan. Heart. 2016;102(17):1342–7.
Prenner SB, Shah SJ, Yancy CW. Role of angiotensin receptor-neprilysin inhibition in heart failure. Curr Atheroscler Rep. 2016;18(8):48.
Feng L, Karpinski PH, Sutton P, et al. LCZ696: a dual-acting sodium supramolecular complex. Tetrahedron Lett. 2012;53(3):275–6.
Gu J, Noe A, Chandra P, et al. Pharmacokinetics and pharmacodynamics of LCZ696, a novel dual-acting angiotensin receptor-neprilysin inhibitor (ARNi). J Clin Pharmacol. 2010;50(4):401–14.
Entresto (sacubitril/valsartan) film-coated tablets: summary of product characteristics. London: European Medicines Agency; 2016.
Entresto™ (sacubitril and valsartan): US prescribing information. East Hanover: Novartis; 2015.
McMurray JJV, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993–1004.
Bohm M, Refsgaard J, Ramires FJA, et al. Effect of the angiotensin receptor neprilysin inhibitor LCZ696 compared with enalapril according to systolic blood pressure in PARADIGM-HF [abstract no. P1794]. Eur J Heart Fail. 2015;17(Suppl 1):393.
Castagno D, Jhund P, Rouleau JL, et al. Prognostic importance of heart rate, and effect of sacubitril/valsartan according to heart rate, in PARADIGM-HF [abstract no. P483]. Eur J Heart Fail. 2016;18(Suppl 1):120–1.
Desai AS, McMurray JJV, Packer M, et al. Effect of the angiotensin-receptor-neprilysin inhibitor LCZ696 compared with enalapril on mode of death in heart failure patients. Eur Heart J. 2015;36(30):1990–7.
Packer M, McMurray JJV, Desai AS, et al. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation. 2014;131(1):54–61.
Okumura N, Jhund PS, Gong J, et al. Importance of clinical worsening of heart failure treated in the outpatient setting: evidence from the prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure trial (PARADIGM-HF). Circulation. 2016;133(23):2254–62.
Desai AS, Claggett BL, Packer M, et al. Influence of sacubitril/valsartan (LCZ696) on 30-day readmission after heart failure hospitalization. J Am Coll Cardiol. 2016;68(3):241–8.
Jhund PS, Fu M, Bayram E, et al. Efficacy and safety of LCZ696 (sacubitril-valsartan) according to age: insights from PARADIGM-HF. Eur Heart J. 2015;36(38):2576–84.
Kristensen SL, Preiss D, Jhund PS, et al. Risk related to pre-diabetes mellitus and diabetes mellitus in heart failure with reduced ejection fraction: insights from prospective comparison of ARNI wth ACEI to determine impact on global mortality and morbidity in heart failure trial. Circ Heart Fail. 2016;9(1):e002560.
Kristensen SL, Martinez F, Jhund PS, et al. Geographic variations in the PARADIGM-HF heart failure trial. Eur Heart J. 2016;. doi:10.1093/eurheartj/ehw226.
Solomon SD, Claggett B, Desai AS, et al. Influence of ejection fraction on outcomes and efficacy of sacubitril/valsartan (LCZ696) in heart failure with reduced ejection fraction: the prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial. Circ Heart Fail. 2016;9(3):e002744.
Simpson J, Jhund PS, Silva Cardoso J, et al. Comparing LCZ696 with enalapril according to baseline risk using the MAGGIC and EMPHASIS-HF risk scores: an analysis of mortality and morbidity in PARADIGM-HF. J Am Coll Cardiol. 2015;66(19):2059–71.
Okumura N, Jhund PS, Gong J, et al. Effects of sacubitril/valsartan in the PARADIGM-HF trial (prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure) according to background therapy. Circ Heart Fail. 2016;9(9). pii: e003212.
Solomon SD, Claggett B, Packer M, et al. Efficacy of sacubitril/valsartan relative to a prior decompensation: the PARADIGM-HF trial. JACC Heart Failure. 2016;4(10):816–22.
Vardeny O, Claggett B, Packer M, et al. Efficacy of sacubitril/valsartan vs. enalapril at lower than target doses in heart failure with reduced ejection fraction: the PARADIGM-HF trial. Eur J Heart Fail. 2016;18(10):1228–34.
Desai AS, Solomon S, Claggett B, et al. Factors associated with noncompletion during the run-in period before randomization and influence on the estimated benefit of LCZ696 in the PARADIGM-HF trial. Circ Heart Fail. 2016;9(6). pii: e002735.
Claggett B, Packer M, McMurray JJV, et al. Estimating the long-term treatment benefits of sacubitril–valsartan. N Engl J Med. 2015;373(23):2289–90.
Cannon J, Boytsov S, Senni M, et al. Dementia-related adverse effects in the prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure trial (PARADIGM-HF) [abstract no. P242]. Eur J Heart Fail. 2015;17(Suppl 1):49–50.
McCormack PL. Sacubitril/valsartan: a review in chronic heart failure with reduced ejection fraction. Drugs. 2016;76(3):387–96.
Acknowledgements
The manuscript was updated from Drugs 2016;76(3):387–96 [27], and was reviewed by: A.L. Clark, Department of Cardiology, Hull York Medical School, Castle Hill Hospital, Hull, UK; P.S. Jhund, British Heart Foundation Cardiovascular Research Centre, Institute of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow, UK; K.S. Kim, Department of Internal Medicine, Daegu Catholic University Medical Center, Daegu, Republic of Korea. During the peer review process, the manufacturer of sacubitril/valsartan was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
G.M. Keating and P.L. McCormack are/were salaried employees of Adis/Springer, are responsible for the article content and declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Keating, G.M., McCormack, P.L. Sacubitril/valsartan in chronic heart failure with reduced ejection fraction: a guide to its use. Drugs Ther Perspect 33, 1–7 (2017). https://doi.org/10.1007/s40267-016-0361-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-016-0361-y