Abstract
Background
Spontaneous reporting of adverse drug reactions (ADRs) remains one of the most efficient methods to detect new, unusual, and severe ADRs. Community pharmacy professionals (CPPs) play a fundamental role in the reporting of spontaneous ADRs. The aim of this study was to describe the attitudes and knowledge of different CPP groups regarding the spontaneous reporting of ADRs and to identify the factors that can influence ADR under-reporting.
Methods
A cross-sectional descriptive study was conducted in CPPs (156 pharmacists and 40 pharmacy technicians) working in 49 pharmacies in Coimbra, Portugal. A survey of the knowledge and attitudes of CPPs towards reporting ADRs and the factors that encourage and discourage ADR reporting was constructed and personally delivered to the pharmacies.
Results
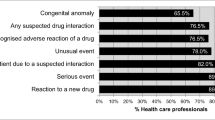
The response rate was 82.0%. The seriousness and the unusualness of the reaction were the most important motives to report ADRs (98.0 and 97.4% of respondents, respectively). CPPs also considered ADR reporting to be a professional obligation (96.4%), but “don’t feel the need to report well-known ADRs” (54.1%). Other attitudes associated with under-reporting were lack of time (50.0%), method of reporting (38.3%), and fear of legal liability (29.6%).
Conclusions
CPPs’ knowledge and behavior play a significant role in ADR reporting. Despite the differences in their educational syllabus, there were no statistical differences between pharmacists and pharmacy technicians with regard to their perception of the importance of ADR reports or the factors that affect their reporting. It may be possible to reduce the under-reporting of ADRs by introducing educational interventions based on the attitudes related to under-reporting that have been identified in this study.
Similar content being viewed by others
References
Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998;279(15):1200–5.
Pirmohamed M, James S, Meakin S, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ. 2004;329(7456):15–9.
Herdeiro MT, Figueiras A, Polónia J, et al. Influence of pharmacists’ attitudes on adverse drug reaction reporting. Drug Saf. 2006;29(4):331–40.
Wester K, Jönsson A, Spigset O, et al. Spontaneously reported fatal suspected adverse drug reactions: a 10-year survey from Sweden. Pharmacoepidemiol Drug Saf. 2007;16(2):173–80.
Dormann H, Muth-Selbach U, Krebs S, et al. Incidence and costs of adverse drug reactions during hospitalisation. Drug Saf. 2000;22(2):161–8.
van Hunsel F, Härmark L, Pal S, et al. Experiences with adverse drug reaction reporting by patients. Drug Saf. 2012;35(1):45–60.
World Health Organization. The importance of pharmacovigilance: safety monitoring of medicinal products. Geneva: World Health Organization; 2002. p. 1–48.
Härmark L, Van Grootheest AC. Pharmacovigilance: methods, recent developments and future perspectives. Eur J Clin Pharmacol. 2008;64(8):743–52.
Hazell L, Cornelius V, Hannaford P, et al. How do patients contribute to signal detection? Drug Saf. 2013;36(3):199–206.
Pal SN, Duncombe C, Falzon D, et al. WHO strategy for collecting safety data in public health programmes: complementing spontaneous reporting systems. Drug Saf. 2013;36(2):75–81.
Lopez-Gonzalez E, Herdeiro MT, Figueiras A. Determinants of under-reporting of adverse drug reactions. Drug Saf. 2009;32(1):19–31.
Hazell L, Shakir SAW. Under-reporting of adverse drug reactions. Drug Saf. 2006;29(5):385–96.
Van Grootheest AC, De Jong-van den Berg LTW. The role of hospital and community pharmacists in pharmacovigilance. Res Soc Admin Pharm. 2005;1(1):126–33.
Mes K, Berg LTW, Grootheest AC. Attitudes of community pharmacists in the Netherlands towards adverse drug reaction reporting. Int J Pharm Pract. 2002;10(4):267–72.
Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients. JAMA. 1998;279(15):1200–5.
van Grootheest K, Olsson S, Couper M, et al. Pharmacists’ role in reporting adverse drug reactions in an international perspective. Pharmacoepidemiol Drug Saf. 2004;13(7):457–64.
Van Grootheest AC, Van Puijenbroek EP, de Jong-van den LTW. Contribution of pharmacists to the reporting of adverse drug reactions. Pharmacoepidemiol Drug Saf. 2002;11(3):205–10.
Urru SAM, Pasina L, Minghetti P, et al. Role of community pharmacists in the detection of potentially inappropriate benzodiazepines prescriptions for insomnia. Int J Clin Pharm. 2015;37(6):1004–8.
Leone R, Moretti U, D’Incau P, et al. Effect of pharmacist involvement on patient reporting of adverse drug reactions: first Italian study. Drug Saf. 2013;36(4):267–76.
Bateman DN, Sanders GL, Rawlins MD. Attitudes to adverse drug reaction reporting in the Northern Region. Br J Clin Pharmacol. 1992;34(5):421.
Belton KJ, Lewis SC, Payne S, et al. Attitudinal survey of adverse drug reaction reporting by medical practitioners in the United Kingdom. Br J Clin Pharmacol. 1995;39(3):223.
Herdeiro MT, Figueiras A, Polonia J, et al. Physicians’ attitudes and adverse drug reaction reporting: a case-control study in Portugal. Drug Saf. 2005;28(9):825–33.
Inman WH. Attitudes to adverse drug reaction reporting. Br J Clin Pharmacol. 1996;41(5):434.
Sweis D, Wong ICK. A survey on factors that could affect adverse drug reaction reporting according to hospital pharmacists in Great Britain. Drug Saf. 2000;23(2):165–72.
Irujo M, Beitia G, Bes-Rastrollo M, et al. Factors that influence under-reporting of suspected adverse drug reactions among community pharmacists in a Spanish region. Drug Saf. 2007;30(11):1073–82.
Toklu HZ, Uysal MK. The knowledge and attitude of the Turkish community pharmacists toward pharmacovigilance in the Kadikoy district of Istanbul. Pharm World Sci. 2008;30(5):556–62.
Belton KJ. Attitude survey of adverse drug-reaction reporting by health care professionals across the European Union. Eur J Clin Pharmacol. 1997;52(6):423–7.
Figueiras A, Tato F, Fontaiñas J, et al. Physicians’ attitudes towards voluntary reporting of adverse drug events. J Eval Clin Pract. 2001;7(4):347–54.
Herdeiro MT, Polonia J, Gestal-Otero JJ, et al. Improving the reporting of adverse drug reactions: a cluster-randomized trial among pharmacists in Portugal. Drug Saf. 2008;31(4):335–44.
Duarte M, Ferreira P, Soares M, et al. Community pharmacists’ attitudes towards adverse drug reaction reporting and their knowledge of the new pharmacovigilance legislation in the southern region of Portugal: a mixed methods study. Drugs Ther Perspect. 2015;31(9):316–22.
Toklu HZ, Soyalan M, Gültekin O, et al. The knowledge and attitude of the healthcare professionals towards pharmacovigilance and adverse drug reaction reporting in Northern Cyprus. J Pharmacovigil. 2016;4:193.
Sencan N, Altinkaynak M, Ferah I, et al. The knowledge and attitudes of physicians and nurses towards adverse event reporting and the effect of pharmacovigilance training: a hospital experience. Hacettepe Univ J Fac Pharm. 2010;30(1):25–40.
Granas AG, Buajordet M, Stenberg-Nilsen H, et al. Pharmacists’ attitudes towards the reporting of suspected adverse drug reactions in Norway. Pharmacoepidemiol Drug Saf. 2007;16(4):429–34.
Figueiras A, Herdeiro MT, Polónia J, et al. An educational intervention to improve physician reporting of adverse drug reactions: a cluster-randomized controlled trial. JAMA. 2006;296(9):1086–93.
Marques J, Ribeiro-Vaz I, Pereira AC, et al. A survey of spontaneous reporting of adverse drug reactions in 10 years of activity in a pharmacovigilance centre in Portugal. Int J Pharm Pract. 2014;22(4):275–82.
Batel-Marques F, Mendes D, Alves C, et al. Pharmacovigilance in Portugal: regional center unit activity [in Portuguese]. Acta Med Port. 2015;28(2):222–32.
Lundin T. Postive trends for VigiBase: 12 million reports & counting. Uppsala Rep. 2016;72:14–5.
INFARMED. National Pharmacovigilance System (SNF): notifications and RAM cases in 2015 [in Portuguese]. Lisbon: INFARMED; 2016.
Gokcekus L, Toklu HZ, Demirdamar R, et al. Dispensing practice in the community pharmacies in the Turkish Republic of Northern Cyprus. Int J Clin Pharm. 2012;34(2):312–24.
Toklu HZ. The pharmacy practice of community pharmacists in Turkey. Marmara Pharm J. 2010;14:53–60.
Johnston R, Saulnier L, Gould O. Best possible medication history in the emergency department: comparing pharmacy technicians and pharmacists. Can J Hosp Pharm. 2010;63(5):359.
Driesenaar JA, De Smet P, van Hulten R, et al. Beliefs about inhaled corticosteroids: comparison of community pharmacists, pharmacy technicians and patients with asthma. J Asthma. 2016;53(10):1051–8.
Acknowledgements
The manuscript has been read and approved by all authors and all authors agreed to the submission of the manuscript to the Journal. The authors would like to thank Clara Rocha, Ph.D. at the Coimbra Health School for her contribution to the data analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Source of funding
No sources of funding were used to assist in the preparation of this manuscript.
Conflict of interest
Cristiano Matos, João Joaquim, and Timóteo Pires have no conflicts of interest that are directly relevant to the content of this study.
Rights and permissions
About this article
Cite this article
Matos, C., Joaquim, J. & Pires, T. Attitudes and knowledge of community pharmacy professionals regarding the spontaneous reporting of adverse drug reactions: a preliminary study in Coimbra, Portugal. Drugs Ther Perspect 33, 88–94 (2017). https://doi.org/10.1007/s40267-016-0355-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-016-0355-9