Abstract
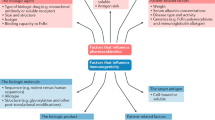
Biologics and biosimilars are biotechnology-derived proteins and have the potential to be highly immunogenic. The consequences of an immune reaction to a therapeutic protein range from transient reactions to severe life-threatening conditions. Patient-, disease- and product-related factors influence the development of immunogenicity, and assessment of immunogenicity is always required for approval of a biosimilar. Assessment of a biologic immunogenicity profile is conducted in equivalence clinical trials to confirm that the immunogenicity profile of the biosimilar is similar to that of its reference biologic. A summary of clinical trials assessing the immunogenicity of infliximab biosimilars in patients with rheumatoid arthritis and ankylosing spondylitis and the results from observational studies in patients with gastroenterology diseases are presented. Due to the small patient populations studied in the equivalence clinical trials, the immune response may not be captured prior to licencing of the biosimilar. Therefore, ongoing pharmacovigillance is required to ensure the safe use of these novel therapies.
Similar content being viewed by others
References
European Medicines Agency (EMA). Guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: non-clinical and clinical issues. December 2014. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/01/WC500180219.pdf. Accessed 12 April 2016.
Therapeutic Goods Administration. Regulation of biosimilar medicines. Australian Government Department of Health. December 2015. https://www.tga.gov.au/publication/evaluation-biosimilars. Accessed 12 April 2016.
Weise M, Bielsky MC, Smet K, et al. Biosimilars: what clinicians should know. Blood. 2012;120(26):5111–7.
European Medicines Agency (EMA). Guideline on immunogenicity assessment of biotechnology-derived therapeutic proteins. September 2015. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/10/WC500194507.pdf. Accessed 12 April 2016.
Baert F, De Vos M, Louis E, et al. Immunogenicity of infliximab: how to handle the problem? Acta Gastroenterol Belg. 2007;70(2):195–202.
U.S. Department of Health and Human Services Food Drug Administration. Guidance for industry. Immunogenicity assessment for therapeutic protein products. August 2014. Available at: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm338856.pdf. Accessed 9 Aug 2016.
Li J, Yang C, Xia Y, Bertino A, et al. Thrombocytopenia caused by the development of antibodies to thrombopoietin. Blood. 2001;98(12):3241–8.
Feagan BC, Choquette D, Ghosh S, et al. The challenge of indication extrapolation for infliximab biosimilars. Biologicals. 2014;42(4):177–83.
U.S. Department of Health and Human Services Food Drug Administration. Scientific considerations in demonstrating biosimilarity to a reference product. April 2015. Available at: http://www.fda.gov/downloads/DrugsGuidanceComplianceRegulatoryInformation/Guidances/UCM291128.pdf. Accessed 8 April 2016.
Park W, Hrycaj P, Jeka S, et al. A randomised, double-blind, multicentre, parallel-group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT-P13 and innovator infliximab in patients with ankylosing spondylitis: the PLANETAS study. Ann Rheum Dis. 2013;72(10):1605–12.
Yoo DH, Hrycaj P, Miranda P, et al. A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study. Ann Rheum Dis. 2013;72(10):1613–20.
Kay J, Smolen JS. Biosimilars to treat inflammatory arthritis: the challenge of proving identity. Ann Rheum Dis. 2013;72(10):1589–93.
Choe JY, Prodanovic N, Niebrzydowski J, et al. A randomized, double-blind, phase III study comparing SB2, an infliximab biosimilar, to the infliximab reference product Remicade in patients with moderate to severe rheumatoid arthritis despite methotrexate therapy. Ann Rheum Dis. 2015. doi:10.1136/annrheumdis-2015-207764.
Park W, Yoo DH, Miranda P, et al. Efficacy and safety of switching from reference infliximab to CT-P13 compared with maintenance of CT-P13 in ankylosing spondylitis: 102-week data from the PLANETAS extension study. Ann Rheum Dis. 2016. doi:10.1136/annrheumdis-2015-208783.
Yoo DH, Prodanovic N, Jaworkski J, et al. Efficacy and safety of CT-P13 (infliximab biosimilar) in patients with rheumatoid arthritis: comparison between switching from reference infliximab to CT-P13 and continuing CT-P13 in the PLANETRA extension study. Ann Rheum Dis. 2016. doi:10.1136/annrheumdis-2015-208786 [Epub 2016].
Gecse KB, Lovász BD, Farkas K, et al. Efficacy and safety of the biosimilar infliximab CT-P13 treatment in inflammatory bowel diseases: a prospective, multicenter, nationwide cohort. J Crohns Colitis. 2016;10(2):133–40.
Jung YS, Park DI, Kim YH, et al. Efficacy and safety of CT-P13, a biosimilar of infliximab, in patents with inflammatory bowel disease: a retrospective multicenter study. J Gastroenterol Hepatol. 2015;30(12):1705–12.
Sieczkowska J, Jarzebicka D, Banaszkiewicz A, et al. Switching between infliximab originator and biosimilar in paediatric patients with inflammatory bowel disease: preliminary observations. J Crohns Colitis. 2016;10(2):127–32.
Ben-Horin S, Heap GA, Ahmad T, et al. The immunogenicity of biosimilar infliximab: can we extrapolate the data across indications? Expert Rev Gastroneterol Hepatol. 2015;9(Suppl 1):27–34.
Pharmaceutical Society of Australia. Biosimilar medicines Position statement. September 2015. https://www.psa.org.au/download/policies/biosimilar-medicines-position-statement.pdf. Accessed on 8 April 2016.
The British Society for Rheumatology. Position statement on biosimilar medicines. London, United Kingdom: the British Society for Rheumatology, February 2015. http://www.rheumatology.org.uk/about_bsr/press_releases/bsr_supports_the_use_of_biolsimilars_but_recommends_measures_to_monitor_safety.aspx. Accessed on 20 April 2016.
Canadian Agency for Drugs and Technologies in Health. Switching from innovator to biosimilar (subsequent entry) infliximab: a review of the clinical effectiveness, cost-effectiveness, and guidelines. Canadian Agency for Drugs and Technologies in Health Rapid Response Report. 2015. Available at: https://www.cadth.ca/media/pdf/htis/mar-2015/RC0635%20Infliximab%20Switching%20Final.pdf. Accessed on 20 April 2016.
Feldman SR. Inflammatory diseases: integrating biosimilars into clinical practice. Semin Arthritis Rheum. 2015;44(6 Suppl):S16–21.
Kurki P. Biosimilars for prescribers. GaBi J. 2015;4(1):33–5.
Ruiz-Argüello B, Maguregui A, Ruiz del Agua A, et al. Antibodies to infliximab in Remicade-treated rheumatic patiens show identical reactivity towards biosimilar CT-P13 [abstract no. OP0015]. Ann Rheum Dis. 2016;75(Suppl 2):58.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mirkov, S., Hill, R. Immunogenicity of biosimilars. Drugs Ther Perspect 32, 532–538 (2016). https://doi.org/10.1007/s40267-016-0341-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-016-0341-2