Abstract
Sumatriptan nasal powder delivered by a breath powered delivery device (ONZETRA® Xsail®) is indicated for the acute treatment of migraine with or without aura in adults. This narrative review discusses the clinical use of sumatriptan nasal powder in this population and summarizes its pharmacological properties. In migraineurs, sumatriptan nasal powder treatment was associated with significantly greater rates of pain relief than placebo from 0.5–2 h postdose after a single treatment in the phase 3 TARGET trial, with these benefits sustained at 24 and 48 h postdose. Compared with oral sumatriptan, sumatriptan nasal powder was associated with significantly faster pain relief during the first 30 min after treatment in the phase 3 COMPASS trial assessing the treatment of up to five migraines in adults and better pain relief and pain freedom from 15 min to 1.5 h postdose. However, there were no between-group differences in pain relief at subsequent timepoints up to 48 h postdose. Sumatriptan nasal powder was generally well tolerated, with the majority of adverse events administration-site related and mild or moderate in severity. In conclusion, sumatriptan nasal powder is an effective and generally well tolerated treatment of migraine. With its novel breath powered nasal delivery resulting in a faster onset of action than oral sumatriptan, sumatriptan nasal powder provides a useful new option for the acute treatment of migraine with and without aura in adults.
Similar content being viewed by others
References
Lipton RB, Stewart WF, Diamond S, et al. Prevalence and burden of migraine in the United States: data from the American migraine study II. Headache. 2001;41(7):646–57.
Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition (beta version). Cephalalgia. 2013;33(9):629–808.
Johnston MM, Rapoport AM. Triptans for the management of migraine. Drugs. 2010;70(12):1505–18.
Cady RJ, Shade CL, Cady RK. Advances in drug development for acute migraine. Drugs. 2012;72(17):2187–205.
Cady R. A novel intranasal breath-powered delivery system for sumatriptan: a review of technology and clinical application of the investigational product AVP-825 in the treatment of migraine. Expert Opin Drug Deliv. 2015;12(9):1565–77.
Obaidi M, Offman E, Messina J, et al. Improved pharmacokinetics of sumatriptan with breath powered nasal delivery of sumatriptan powder. Headache. 2013;53(8):1323–33.
Perry CM, Markham A. Sumatriptan: an updated review of its use in migraine. Drugs. 1998;55(6):889–922.
Villalon CM, Centurion D, Valdivia LF, et al. Migraine: pathophysiology, pharmacology, treatment and future trends. Curr Vasc Pharmacol. 2003;1(1):71–84.
Avanir Pharmaceuticals Inc. ONZETRA® Xsail® (sumatriptan nasal powder): US prescribing information. 2016. https://www.onzetra.com/. Accessed 8 Sep 2016.
Cady RK, McAllister PJ, Spierings EL, et al. A randomized, double-blind, placebo-controlled study of breath powered nasal delivery of sumatriptan powder (AVP-825) in the treatment of acute migraine (The TARGET Study). Headache. 2015;55(1):88–100.
Tepper SJ, Cady RK, Silberstein S, et al. AVP-825 breath-powered intranasal delivery system containing 22 mg sumatriptan powder vs 100 mg oral sumatriptan in the acute treatment of migraines (The COMPASS study): a comparative randomized clinical trial across multiple attacks. Headache. 2015;55(5):621–35.
Djupesland PG, Docekal P. Intranasal sumatriptan powder delivered by a novel breath-actuated bi-directional device for the acute treatment of migraine: a randomised, placebo-controlled study. Cephalalgia. 2010;30(8):933–42.
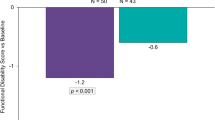
McAllister PJ, Cady RK, Spierings ELH, et al. Breath-poweredTM nasal delivery of powdered sumatriptan (AVP-825): migraine disability and functional outcome in a phase 3 study (TARGET) [Poster no. P42]. Headache. 2014;54(Suppl S1):32–3.
Winner P, Wallick C, Shulman KJ, et al. Rapid response in migraine patients treated with breath powered intranasal delivery of sumatriptan powder (AVP-825): efficacy analysis by prior triptan history from the phase 3 TARGET study [Poster no. P6.102]. Neurology. 2016;86(16 Suppl).
Halker R, Tepper S, Shulman KJ, et al. Total migraine freedom for breath powered intranasal delivery system containing 22 mg sumatriptan powder (AVP-825) vs 100 mg oral sumatripta from the COMPASS study of acute treatment of migraine [Poster no. P6.098]. Neurology. 2016;86(16 Suppl).
Munakata J, Hazard E, Serrano D, et al. Economic burden of transformed migraine: results from the American Migraine Prevalence and Prevention (AMPP) Study. Headache. 2009;49(4):498–508.
Lipton RB, Silberstein SD. Episodic and chronic migraine headache: breaking down barriers to optimal treatment and prevention. Headache. 2015;55(Suppl 2):103–22.
Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: the American Headache Society evidence assessment of migraine pharmacotherapies. Headache. 2015;55(1):3–20.
Bigal M, Rapoport A, Aurora S, et al. Satisfaction with current migraine therapy: experience from 3 centers in US and Sweden. Headache. 2007;47(4):475–9.
Cady RK, Silberstein SD, Tepper SJ. Translating migraine research into clinical practice: practical expert perscpectives. 2015. http://www.medscape.org/. Accessed 27 May 2016.
Cady R. The pharmacokinetics and clinical efficacy of AVP-825: a potential advancement for acute treatment of migraine. Expert Opin Pharmacother. 2015;16(13):2039–51.
Data on file. Avanir Pharmaceuticals Inc.; 2016.
Acknowledgments
During the peer review process, the manufacturer of sumatriptan nasal powder was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
Zaina T. Al-Salama and Lesley J. Scott are salaried employees of Adis/Springer, are responsible for the article content and declare no relevant conflicts of interest.
Additional information
The manuscript was reviewed by: J.R. Couch Jr, Department of Neurology, University of Oklahoma Medical School, Oklahoma city, OK, USA; A.W. Fox, Faculty of Life Sciences and Medicine, Institute of Pharmaceutical Sciences, King’s College London, London, UK; M.J. Marmura, Department of Neurology, Thomas Jefferson University, Philadelphia, PA, USA.
Rights and permissions
About this article
Cite this article
Al-Salama, Z.T., Scott, L.J. Sumatriptan Nasal Powder: A Review in Acute Treatment of Migraine. Drugs 76, 1477–1484 (2016). https://doi.org/10.1007/s40265-016-0641-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-016-0641-9