Abstract
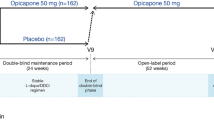
Oral opicapone (Ongentys®), a potent, third-generation, long-acting, peripheral catechol-O-methyltransferase (COMT) inhibitor, is approved as adjunctive treatment to levodopa (L-Dopa)/dopa-decarboxylase inhibitor (DDCI) therapy in adults with Parkinson’s disease (PD) and end-of-dose motor fluctuations who cannot be stabilized on those combinations. In 14- to 15-week, double-blind, multinational trials and in 1-year, open-label extension studies in this patient population, opicapone was an effective and generally well tolerated adjunctive therapy to L-Dopa plus a DDCI and other PD therapy. During the double-blind phase, adjunctive opicapone 50 mg once daily provided significantly greater improvements in motor fluctuations than placebo, with these improvements noninferior to those with entacapone. These beneficial improvements in motor fluctuations with opicapone were maintained in patients who continued adjunctive opicapone during the extension studies, with patients who switched from placebo or entacapone to opicapone experiencing significant improvements in motor fluctuations during this year. No new unexpected safety concerns were identified after ≈1.4 years’ treatment with opicapone, with no serious cases of hepatotoxicity reported in clinical trials. With its convenient once-daily regimen, oral opicapone is an emerging COMT inhibitor option for use as adjunctive therapy to L-Dopa/DDCI therapy in adults with PD and end-of dose motor fluctuations who cannot be stabilized on those combinations.
Similar content being viewed by others
Change history
21 November 2017
Parkinson’s disease (PD) is the most common chronic, progressive, neurodegenerative disease, with a mean age of onset of 57 years [1, 2].
03 September 2016
An erratum to this article has been published.
References
Samii A, Nutt J, Ransom RR, et al. Parkinson’s disease. Lancet. 2004;2004(362):1783–93.
LeWitt PA. Levodopa for the treatment of Parkinson’s disease. N Engl J Med. 2008;359(23):2468–76.
Müller T. Catechol-O-methyltransferase inhibitors in Parkinson’s disease. Drugs. 2015;75(2):157–74.
European Medicines Agency. Ongentys 25 mg hard capsules: summary of product characteristics. 2016. http://www.ema.europa.eu. Accessed 12 July 2016.
Kiss LE, Ferreira HS, Torrao L, et al. Discovery of a long-acting, peripherally selective inhibitor of catechol-O-methyltransferase. J Med Chem. 2010;53(8):3396–411.
Bonifacio MJ, Torrao L, Loureiro AI, et al. Pharmacological profile of opicapone, a third-generation nitrocatechol catechol-O-methyl transferase inhibitor, in the rat. Br J Pharmacol. 2015;172(7):1739–52.
Bonifacio MJ, Sutcliffe JS, Torrao L, et al. Brain and peripheral pharmacokinetics of levodopa in the cynomolgus monkey following administration of opicapone, a third generation nitrocatechol COMT inhibitor. Neuropharmacology. 2014;77:334–41.
Almeida L, Rocha JF, Falcao A, et al. Pharmacokinetics, pharmacodynamics and tolerability of opicapone, a novel catechol-O-methyltransferase inhibitor, in healthy subjects: prediction of slow enzyme-inhibitor complex dissociation of a short-living and very long-acting inhibitor. Clin Pharmacokinet. 2013;52(2):139–51.
Rocha JF, Almeida L, Falcao A, et al. Opicapone: a short lived and very long acting novel catechol-O-methyltransferase inhibitor following multiple dose administration in healthy subjects. Br J Clin Pharmacol. 2013;76(5):763–75.
Falcão A, Rocha JF, Santos A, et al. Opicapone pharmacokinetics and pharmacodynamics comparison between healthy Japanese and matched white subjects. Clin Pharmacol Drug Dev. 2016;5(2):150–61.
Ferreira JJ, Rocha JF, Falcao A, et al. Effect of opicapone on levodopa pharmacokinetics, catechol-O-methyltransferase activity and motor fluctuations in patients with Parkinson’s disease. Eur J Neurol. 2015;22(5):815-25, e56.
Rocha J-F, Ferreira JJ, Falcao A, et al. Effect of 3 single-dose regimens of opicapone on levodopa pharmacokinetics, catechol-O-methyltransferase activity and motor response in patients with Parkinson disease. Clin Pharmacol Drug Dev. 2015;5(3):232–40.
Rocha JF, Falcao A, Santos A, et al. Effect of opicapone and entacapone upon levodopa pharmacokinetics during three daily levodopa administrations. Eur J Clin Pharmacol. 2014;70:1059–71.
Pinto R, l’Hostis P, Patat A, et al. Evaluation of opicapone on cardiac repolarization in a thorough QT/QTc study. Clin Pharmacol Drug Develop. 2015;4(6):454–62.
Rocha JF, Santos A, Falcao A, et al. Effect of moderate liver impairment on the pharmacokinetics of opicapone. Eur J Clin Pharmacol. 2014;70(3):279–86.
Ferreira JJ, Lees A, Rocha JF, et al. Opicapone as an adjunct to levodopa in patients with Parkinson’s disease and end-of-dose motor fluctuations: a randomised, double-blind, controlled trial. Lancet Neurol. 2016;15(2):154–65.
Bial-Portela & Ca, S.A. NCT01227655. 2015. http://www.clinicaltrials.gov/. Accessed 12 Jul 2016.
Lees A, Ferreira JJ, Costa R, et al. Efficacy and safety of opicapone, a new COMT-inhibitor, for the treatment of motor fluctuations in Parkinson’s disease patients: BIPARK-II study [abstract no. 1038]. J Neurol Sci. 2013;333(Suppl 1):e116.
Oliveira C, Lees A, Ferreira J, et al. Opicapone and non-motor symptoms in Parkinson’s disease: results from a double-blind, randomized, placebo-controlled study and open-label extension. Mov Disord. 2015;30(Suppl 1):S173.
Ferreira J, Lees A, Santos A, et al. Efficacy of opicapone as adjunctive therapy to levodopa in patients with Parkinson’s disease and motor fluctuations: analysis of pooled phase III studies. Mov Disord. 2015;30(Suppl 1):S86.
Lees A, Ferreira J, Lopes N, et al. Efficacy and safety of opicapone in patients over 70 years with Parkinsons disease and motor fluctuations. Mov Disord. 2015;30(Suppl 1):S99.
Lopes N, Ferreira J, Lees A, et al. Exploratory efficacy of opicapone in combination with dopamine agonists or MAO-B inhibitors on the treatment of motor fluctuations in Parkinsons disease. Mov Disord. 2015;30(Suppl 1):S101.
Lees A, Ferreira J, Muller T, et al. Opicapone in fluctuating Parkinson’s disease patients: OFF- and ON-time responder post hoc analyses of pooled phase III studies [abstract no. 1953]. Mov Disord. 2016;31(Suppl 2):S642.
Lees A, Ferreira J, Reichmann H, et al. Onset and stabilization of treatment effects in fluctuating Parkinson’s disease patients: exploratory by-week efficacy analysis of pooled phase III studies [abstract no. 1954]. Mov Disord. 2016;31(Suppl 2):S642.
Lopes N, Ferreira J, Lees A, et al. Exploratory efficacy of opicapone in fluctuating Parkinson’s disease patients at different stages of symptom progression [abstract no. 1955]. Mov Disord. 2016;31(Suppl 2):S642.
Ferreira J, Lees A, Tolosa E, et al. Switching double-blind opicapone, entacapone or placebo to open-label opicapone: efficacy results of the 1-year extension of study BIPARK I [abstract no. 1925]. Mov Disord. 2016;31(Suppl 2):S633.
Ferreira J, Lees A, Rascol O, et al. Activities of daily living and motor scores of the UPDRS in fluctuating Parkinson’s disease treated with opicapone [abstract no. 1956]. Mov Disord. 2016;31(Suppl 2):S643.
Costa R, Oliveira C, Pinto R, et al. One-year open-label efficacy and safety of opicapone in Parkinson’s disease BIPARK-II study. Mov Disord. 2014;29(Suppl 1):S233.
Ferreira J, Lees A, Gama H, et al. Safety and tolerability of opicapone in the treatment of Parkinson’s disease and motor fluctuations: analysis of pooled phase III studies. Mov Disord. 2015;30(Suppl 1):S86.
Lees A, Ferreira J, Costa R, et al. 1-year safety of opicapone in patients with Parkinson’s disease and motor fluctuations [abstract no. 257]. Mov Disord. 2015;30(Suppl 1):S99.
Lopes N, Ferreira J, Lees A, et al. Hepatic safety of opicapone in Parkinson’s disease patients. Mov Disord. 2015;30(Suppl 1):S101.
Lopes N, Ferreira J, Lees A, et al. Evaluation of impulse of control disorders in fluctuating Parkinson’s disease patients under opicapone treatment [abstract no. 1957]. Mov Disord. 2016;31(Suppl 2):S643.
Pinto R, Vaz-da-Silva M, Lopes N, et al. Cardiac safety of opicapone in patients with Parkinson’s disease: analysis of the centralized phase III ECG dataset. Mov Disord. 2015;30(Suppl 1):S112.
Santos A, Ferreira J, Lees A, et al. Safety of opicapone in fluctuating Parkinson’s disease patients: results of the 1-year extension of study BIPARK I [abstract no. 1949]. Mov Disord. 2016;31(Suppl 2):641.
Goetz CG, Pal G. Initial management of Parkinson’s disease. Br Med J. 2014;349:g6258.
Ferreira JJ, Katzenschlager R, Bloem BR, et al. Summary of the recommendations of the EFNS/MDS-ES review on therapeutic management of Parkinson’s disease. Eur J Neurol. 2013;20:5–15.
Clarke CE. Parkinson’s disease. Br Med J. 2007;335:441–5.
Dorsey ER, Constantinescu R, Thompson JP, et al. Projected number of people with Parkinson’s disease in the most populous nations, 2005 through 2030. Neurology. 2007;68(5):384–6.
National Institute for Health and Clinical Excellence. Parkinson’s disease in over 20 s: diagnosis and management. 2006. http://www.nice.org.uk/guidance/cg35. Accessed 22 June 2016.
European Medicines Agency. Stalevo 50 mg/12.5 mg/200 mg film-coated tablets: summary of product characteristics 2008. http://www.ema.europa.eu. Accessed 24 June 2016.
Acknowledgments
During the peer review process, the manufacturer of opicapone was offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflicts of interest
Lesley Scott is a salaried employee of Adis/Springer, is responsible for the article content and declares no relevant conflicts of interest.
Additional information
The manuscript was reviewed by: A. Antonini, Parkinson’s Disease and Movement Disorders Unit, IRCCS Hospital San Camillo Venice, 1st Neurology Clinic, Padua University Hospital, Padua, Italy; J. Fernandez - Ruiz, Facultad de Medicina Departamento de Bioquimica y Biologia Molecular III, Instituto Universitario de Investigacion en Neuroquimica, Madrid, Spain; P. J. Garcia Ruiz, Department of Neurology, Fundacion Jimenez Diaz, Madrid, Spain; K. Jellinger, Institute of Clinical Neurobiology, Vienna, Austria; M. G. Şenol, Department of Neurology, GATA Haydarpaşa Training Hospital, Istanbul, Turkey.
A correction to this article is available online at https://doi.org/10.1007/s40265-017-0847-5.
An erratum to this article is available at https://doi.org/10.1007/s40265-016-0637-5.
Rights and permissions
About this article
Cite this article
Scott, L.J. Opicapone: A Review in Parkinson’s Disease. Drugs 76, 1293–1300 (2016). https://doi.org/10.1007/s40265-016-0623-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-016-0623-y