Abstract
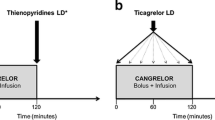
Cangrelor (Kengrexal®, Kengreal™) is an intravenously administered P2Y12 receptor inhibitor. It is direct-acting and reversible, with a very rapid onset and offset of action. The randomized, double-blind, multinational, phase III CHAMPION PHOENIX trial compared the efficacy of intravenous cangrelor with that of oral clopidogrel in patients requiring percutaneous coronary intervention (PCI) for stable angina pectoris, a non-ST-segment elevation acute coronary syndrome or ST-segment elevation myocardial infarction (MI). The primary composite efficacy endpoint of death from any cause, MI, ischaemia-drive revascularization or stent thrombosis in the 48 h following randomization occurred in significantly fewer cangrelor than clopidogrel recipients. The rate of severe or life-threatening non-coronary artery bypass graft-related, GUSTO-defined bleeding at 48 h did not significantly differ between cangrelor and clopidogrel recipients. In conclusion, intravenous cangrelor is an important new option for use in patients undergoing PCI who have not been treated with oral P2Y12 inhibitors.
Similar content being viewed by others
References
Wallentin L. P2Y12 inhibitors: differences in properties and mechanisms of action and potential consequences for clinical use. Eur Heart J. 2009;30(16):1964–77.
Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124(23):e574–651.
O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary. A report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation. 2013;127(4):529–55.
Windecker S, Kolh P, Alfonso F, et al. 2014 ESC/EACTS guidelines on myocardial revascularization: the task force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;35(37):2541–619.
Lhermusier T, Baker NC, Waksman R. Overview of the 2014 Food and Drug Administration Cardiovascular and Renal Drugs Advisory Committee meeting regarding cangrelor. Am J Cardiol. 2015;115(8):1154–61.
Waite LH, Phan YL, Spinler SA. Cangrelor: a novel intravenous antiplatelet agent with a questionable future. Pharmacotherapy. 2014;34(10):1061–76.
European Medicines Agency. Kengrexal (cangrelor): EU summary of product characteristics. 2015. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003773/WC500188098.pdf. Accessed 15 June 2015.
The Medicines Company. Kengreal™ (cangrelor) for injection, for intravenous use: US prescribing information. 2015. http://www.kengreal.com/pdfs/kengreal-us-prescribing-information.pdf. Accessed 24 June 2015.
Xiang B, Zhang G, Ren H, et al. The P2Y12 antagonists, 2MeSAMP and cangrelor, inhibit platelet activation through P2Y12/Gi-dependent mechanism. PLoS One. 2012;7(12):e51037.
Akers WS, Oh JJ, Oestreich JH, et al. Pharmacokinetics and pharmacodynamics of a bolus and infusion of cangrelor: a direct, parenteral P2Y12 receptor antagonist. J Clin Pharmacol. 2010;50(1):27–35.
Storey RF, Wilcox RG, Heptinstall S. Comparison of the pharmacodynamic effects of the platelet ADP receptor antagonists clopidogrel and AR-C69931MX in patients with ischaemic heart disease. Platelets. 2002;13(7):407–13.
Storey RF, Oldroyd KG, Wilcox RG. Open multicentre study of the P2T receptor antagonist AR-C69931MX assessing safety, tolerability and activity in patients with acute coronary syndromes. Thromb Haemost. 2001;85(3):401–7.
Greenbaum AB, Grines CL, Bittl JA, et al. Initial experience with an intravenous P2Y12 platelet receptor antagonist in patients undergoing percutaneous coronary intervention: results from a 2-part, phase II, multicenter, randomized, placebo- and active-controlled trial. Am Heart J. 2006;151(3):689 e1–e10.
Ingall AH, Dixon J, Bailey A, et al. Antagonists of the platelet P2T receptor: a novel approach to antithrombotic therapy. J Med Chem. 1999;42(2):213–20.
Srinivasan S, Mir F, Huang J-S, et al. The P2Y12 antagonists, 2-methylthioadenosine 5′-monophosphate triethylammonium salt and cangrelor (ARC69931MX), can inhibit human platelet aggregation through a Gi-independent increase in cAMP levels. J Biol Chem. 2009;284(24):16108–17.
Ferreiro J, Ueno M, Tello-Montoliu A, et al. Effects of cangrelor in coronary artery disease patients with and without diabetes mellitus: an in vitro pharmacodynamic investigation. J Thromb Thrombolysis. 2013;35(2):155–64.
Angiolillo DJ, Schneider DJ, Bhatt DL, et al. Pharmacodynamic effects of cangrelor and clopidogrel: the platelet function substudy from the cangrelor versus standard therapy to achieve optimal management of platelet inhibition (CHAMPION) trials. J Thromb Thrombolysis. 2012;34(1):44–55.
Steinhubl SR, Oh JJ, Oestreich JH, et al. Transitioning patients from cangrelor to clopidogrel: pharmacodynamic evidence of a competitive effect. Thromb Res. 2008;121(4):527–34.
Green CL, Whellan DJ, Lambe L, et al. Electrocardiographic safety of cangrelor, a new intravenous antiplatelet agent: a randomized, double-blind, placebo- and moxifloxacin-controlled thorough QT study. J Cardiovasc Pharmacol. 2013;62(5):466–78.
Dovlatova NL, Jakubowski JA, Sugidachi A, et al. The reversible P2Y12 antagonist cangrelor influences the ability of the active metabolites of clopidogrel and prasugrel to produce irreversible inhibition of platelet function. J Thromb Haemost. 2008;6(7):1153–9.
Schneider DJ, Seecheran N, Raza SS, et al. Pharmacodynamic effects during the transition between cangrelor and prasugrel. Coron Artery Dis. 2015;26(1):42–8.
Schneider DJ, Agarwal Z, Seecheran N, et al. Pharmacodynamic effects during the transition between cangrelor and ticagrelor. JACC Cardiovasc Interv. 2014;7(4):435–42.
Harrington RA, Stone GW, McNulty S, et al. Platelet inhibition with cangrelor in patients undergoing PCI. N Engl J Med. 2009;361(24):2318–29.
Bhatt DL, Lincoff AM, Gibson CM, et al. Intravenous platelet blockade with cangrelor during PCI. N Engl J Med. 2009;361(24):2330–41.
Leonardi S, Mahaffey KW, White HD, et al. Rationale and design of the Cangrelor versus standard therapy to acHieve optimal Management of Platelet InhibitiON PHOENIX trial. Am Heart J. 2012;163(5):768–76.e2.
Leonardi S, Truffa AAM, Neely ML, et al. A novel approach to systematically implement the universal definition of myocardial infarction: insights from the CHAMPION PLATFORM trial. Heart. 2013;99(17):1282–7.
Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Circulation. 2012;126(16):2020–35.
Thygesen K, Alpert JS, White HD, et al. Universal definition of myocardial infarction. Circulation. 2007;116(22):2634–53.
White HD, Chew DP, Dauerman HL, et al. Reduced immediate ischemic events with cangrelor in PCI: a pooled analysis of the CHAMPION trials using the universal definition of myocardial infarction. Am Heart J. 2012;163(2):182–90 e4.
Bhatt DL, Stone GW, Mahaffey KW, et al. Effect of platelet inhibition with cangrelor during PCI on ischemic events. N Engl J Med. 2013;368(14):1303–13.
Généreux P, Stone GW, Harrington RA, et al. Impact of intraprocedural stent thrombosis during percutaneous coronary intervention: insights from the CHAMPION PHOENIX Trial (clinical trial comparing cangrelor to clopidogrel standard of care therapy in subjects who require percutaneous coronary intervention). J Am Coll Cardiol. 2014;63(7):619–29.
White HD, Bhatt DL, Gibson CM, et al. Outcomes with cangrelor versus clopidogrel on a background of bivalirudin: insights from the CHAMPION PHOENIX (a clinical trial comparing cangrelor to clopidogrel standard therapy in subjects who require percutaneous coronary intervention [PCI]). JACC Cardiovasc Interv. 2015;8(3):424–33.
O’Donoghue ML, Bhatt DL, Stone G, et al. The efficacy, safety, and net clinical benefit of cangrelor in women undergoing elective or urgent PCI: insights from the CHAMPION-PHOENIX trial [abstract no. 1139-083]. J Am Coll Cardiol. 2015;65(10 Suppl):A104.
Cavender M, Bhatt D, Stone G, et al. Cangrelor in elderly patients endergoing PCI: findings from CHAMPION-PHOENIX [abstract no. 2101-286 ]. J Am Coll Cardiol. 2015;65(10 Suppl):A1773.
Steg PG, Bhatt DL, Hamm CW, et al. Effect of cangrelor on periprocedural outcomes in percutaneous coronary interventions: a pooled analysis of patient-level data. Lancet. 2013;382(9909):1981–92.
Angiolillo DJ, Bhatt DL, Steg PG, et al. Impact of cangrelor overdosing on bleeding complications in patients undergoing percutaneous coronary intervention: insights from the CHAMPION trials. J Thromb Thrombolysis. 2015;. doi:10.1007/s11239-015-1233-3.
Bristol-Myers Squibb/Sanofi. Plavix (clopidogrel bisulfate) tablets: US prescribing information. 2013. http://www.bms.com/. Accessed 16 Apr 2015.
Eli Lilly and Company. Effient (prasugrel) tablets for oral use: US prescribing information. 2013. http://www.effient.com/. Accessed 16 Apr 2015.
AstraZeneca. Brilinta® (ticagrelor) tablets for oral use: US prescribing information. 2015. http://www.brilinta.com/. Accessed 16 Apr 2015.
Parodi G, Bellandi B, Xanthopoulou I, et al. Morphine is associated with a delayed activity of oral antiplatelet agents in patients with ST-elevation acute myocardial infarction undergoing primary percutaneous coronary intervention. Circ Cardiovasc Interv. 2015;8(1):e001593.
Lange RA, Hillis LD. The duel between dual antiplatelet therapies. N Engl J Med. 2013;368(14):1356–7.
European Medicines Agency. Plavix (clopidogrel): EU summary of product characteristics. 2015. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000174/WC500042189.pdf. Accessed 29 June 2015.
European Medicines Agency. Kengrexal (cangrelor): EU public assessment report. 2015. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/003773/WC500188100.pdf. Accessed 15 June 2015.
Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045–57.
Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357(20):2001–15.
European Medicines Agency. Brilique (ticagrelor): EU summary of product characteristics. 2014. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001241/WC500100494.pdf. Accessed 29 June 2015.
European Medicines Agency. Efient (prasugrel): EU summary of product characteristics. 2014. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000984/WC500021971.pdf. Accessed 29 June 2015.
Tamborini Permunian E, Riva N, Guasti L, et al. Cangrelor for the treatment of arterial thrombosis: Pharmacokinetics/pharmacodynamics and clinical data. Expert Opin Drug Metab Toxicol. 2015;11(4):625–37.
Angiolillo DJ, Firstenberg MS, Price MJ, et al. Bridging antiplatelet therapy with cangrelor in patients undergoing cardiac surgery: a randomized controlled trial. JAMA. 2012;307(3):265–74.
Firstenberg MS, Dyke CM, Angiolillo DJ, et al. Safety and efficacy of cangrelor, an intravenous, short-acting platelet inhibitor in patients requiring coronary artery bypass surgery. Heart Surg Forum. 2013;16(2):61–70.
Food and Drug Administration. FDA briefing document for the cardiovascular and renal drugs advisory committee (CRDAC): cangrelor injection. 2014. http://www.fda.gov. Accessed 1 Apr 2015.
Food and Drug Administration. FDA briefing document for the cardiovascular and renal drugs advisory committee (CRDAC): Kengreal (cangrelor) for injection. 2015. http://www.fda.gov. Accessed 14 Apr 2015.
Disclosure
The preparation of this review was not supported by any external funding. During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on this article. Changes resulting from comments received were made by the author on the basis of scientific and editorial merit. Gillian Keating is a salaried employee of Adis/Springer.
Author information
Authors and Affiliations
Corresponding author
Additional information
The manuscript was reviewed by: S. Leonardi, Department of Cardiology, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy; A. Mandurino-Mirizzi, University of Pavia, Pavia, Italy; D. J. Schneider, Cardiology Unit, Cardiovascular Research Institute, Department of Medicine, University of Vermont, Colchester, VT, USA.
Rights and permissions
About this article
Cite this article
Keating, G.M. Cangrelor: A Review in Percutaneous Coronary Intervention. Drugs 75, 1425–1434 (2015). https://doi.org/10.1007/s40265-015-0445-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-015-0445-3