Abstract
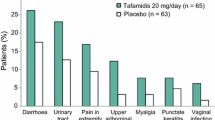
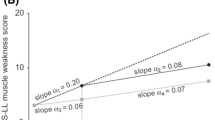
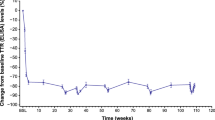
Oral tafamidis (Vyndaqel®) is indicated in the EU for the treatment of transthyretin (TTR) amyloidosis in adult patients with early stage symptomatic polyneuropathy to delay peripheral neurologic impairment and, in Argentina, Japan and Mexico, for delaying the peripheral neurological impairment of TTR familial amyloid polyneuropathy (TTR-FAP). It is the first disease-modifying pharmacotherapy to be approved for use in adult patients with early-stage TTR-FAP. The drug acts to kinetically stabilize the variant TTR tetramer and thereby prevent tetramer dissociation, the rate-limiting step in TTR misfolding and amyloidogenesis. In the 18-month Fx-005 study in adult patients with early-stage V30M TTR-FAP, there were no statistically significant differences between tafamidis and placebo recipients for the coprimary endpoints, the Neuropathy Impairment Score-Lower Limb (NIS-LL) response rate (increase in NIS-LL score of <2) and the least-square mean change in Norfolk Quality-of-Life (QOL) Diabetic-Neuropathy Questionnaire total scores at 18 months, based on modified intent-to-treat analyses. However, in prespecified per-protocol analyses of efficacy evaluable patients, tafamidis recipients experienced significantly better outcomes in terms of these coprimary endpoints, with most (98 %) tafamidis recipients showing stabilization of TTR tetramers at study end. Secondary endpoint outcomes also favoured tafamidis treatment over placebo. The beneficial effects of tafamidis in slowing deterioration of neurological function and health-related-QOL were maintained in long-term extension studies (up to 66 months). Tafamidis also stabilized TTR tetramers in patients with non-V30M TTR-FAP. Tafamidis was generally well tolerated, with most treatment-emergent adverse events being of mild to moderate intensity and very few patients discontinuing treatment because of these events. No new safety signals have emerged during long-term extension studies and post-marketing experience.




Similar content being viewed by others
References
Ando Y, Coelho T, Berk JL, et al. Guideline of transthyretin-related hereditary amyloidosis for clinicians. Orphanet J Rare Dis. 2013;8:31.
Hou X, Aguilar M-I, Small DH. Transthyretin and familial amyloidotic polyneuropathy: recent progress in understanding the molecular mechanism of neurodegeneration. FEBS J. 2007;274(7):1637–50.
Planté-Bordeneuve V, Said G. Familial amyloid polyneuropathy. Lancet Neurol. 2011;10(12):1086–97.
Adams D, Théaudin M, Cauquil C, et al. FAP neuropathy and emerging treatments. Curr Neurol Neurosci Rep. 2014;14(3):435.
Merlini G, Planté-Bordeneuve V, Judge DP, et al. Effects of tafamidis on transthyretin stabilization and clinical outcomes in patients with non-Val30Met transthyretin amyloidosis. J Cardiovasc Transl Res. 2013;6(6):1011–20.
Nencetti S, Rossello A, Orlandini E, et al. Tafamidis (Vyndaquel): a light for FAP patients. ChemMedChem. 2014;8(10):1617–9.
Sekijima Y. Recent progress in the understanding and treatment of transthyretin amyloidosis. Clin Pharm Ther. 2014;39(3):225–33.
Bulawa CE, Connelly S, DeVit M, et al. Tafamidis, a potent and selective transthyretin kinetic stabilizer that inhibits the amyloid cascade. Proc Natl Acad Sci USA. 2012;109(24):9629–34.
Penchala SC, Connelly S, Wang Y, et al. AG10 inhibits amyloidogenesis and cellular toxicity of the familial amyloid cardiomyopathy-associated V122I transthyretin. Proc Natl Acad Sci USA. 2013;110(24):9992–7.
European Medicines Agency. Vyndaqel (tafamidis) 20 mg soft capsules: summary of product characteristics. 2013. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002294/human_med_001498.jsp&mid=WC0b01ac058001d124. Accessed 5 May 2014.
Coelho T, Maia LF, da Martins Silva A, et al. Tafamidis for transthyretin familial amyloid polyneuropathy: a randomized, controlled trial. Neurology. 2012;79(8):785–92.
Coelho T, Maia LF, da Martins Silva A, et al. Long-term effects of tafamidis for the treatment of transthyretin familial amyloid polyneuropathy. J Neurol. 2013;260(11):2802–14.
Coelho T, Silva A, Maia L, et al. Long-term use of tafamidis in transthyretin familial amyloid polyneuropathy: a single center experience [abstract No. S58.005]. Neurology. 2013;80(1 Meeting Abstracts).
Coelho T, Conceição IM, Barroso F, et al. Long term effects of tafamidis treatment on transthyretin familial amyloid polyneuropathy (TTR-FAP): interim results of the Fx1A-303 study [abstract OS3114]. Eur J Neurol. 2014;21(Suppl 1):81.
Coelho T, Silva AM, Valdrez K, et al. Familial amyloid polyneuropathy treatment with tafamidis: evaluation of one year treatment in Porto, Portugal [abstract No. OP-67]. XIVth international symposium on amyloidosis; Apr 27-May 1 2014; Indianapolis, IN.
Damy T, Judge DP, Dogan A, et al. Cardiac safety and tolerability, and effects on cardiac function, of tafamidis in patients with non-V30M TTR-FAP [abstract No. P888]. Eur J Heart Failure. 2012;11(Suppl):S153–4.
Pfizer Inc. Familial amyloidosis support meeting. 2013. http://www.amyloidosissupport.org/13fam_pfizer.pdf. Accessed 5 May 2014.
Pfizer Inc. FDA issues complete response letter for Pfizer’s tafamidis meglumine new drug application. 2012. http://www.pfizer.com/news/press-release/press-release-archive-detail/fda_issues_complete_response_letter_for_pfizer_s_tafamidis_meglumine_new_drug_application. Accessed 5 May 2014.
Disclosure
The preparation of this review was not supported by any external funding. During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on this article. Changes resulting from comments received were made by the authors on the basis of scientific and editorial merit. Lesley Scott is a salaried employee of Adis/Springer.
Author information
Authors and Affiliations
Corresponding author
Additional information
The manuscript was reviewed by: T. Coelho, Unidade Clinica de Paramiloidose, Hospital de Santo Antonio, Centro Hospitalar do Porto, Porto, Portugal; G. Merlini, Amyloid Research and Treatment Center, IRCSS Fondazione Policlinico San Matteo, Department of Molecular Medicine, University of Pavia, Pavia, Italy.
Rights and permissions
About this article
Cite this article
Scott, L.J. Tafamidis: A Review of Its Use in Familial Amyloid Polyneuropathy. Drugs 74, 1371–1378 (2014). https://doi.org/10.1007/s40265-014-0260-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-014-0260-2