Abstract
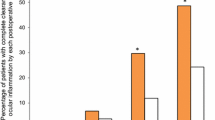
Loteprednol etabonate ophthalmic gel 0.5 % (Lotemax®) is approved in the USA for the treatment of post-operative inflammation and pain in patients who have undergone ocular surgery. The new gel formulation of loteprednol etabonate offers some potential advantages over the previously available ophthalmic suspension and ointment formulations of the drug. Because the gel is non-settling, a uniform dose of loteprednol etabonate is delivered without the need to vigorously shake the product. The pH of the gel formulation is close to that of physiological tears and the concentration of preservative is low. In clinical trials, loteprednol etabonate ophthalmic gel 0.5 % for 14 days was effective, very well tolerated and safe when used for the treatment of post-operative inflammation and pain following cataract surgery. Relative to vehicle, loteprednol etabonate ophthalmic gel 0.5 % effectively reduced postoperative ocular inflammation and ocular pain and had a similar overall tolerability, comfort and safety profile. It is associated with a low risk of inducing clinically significant increases in intraocular pressure. In conclusion, loteprednol etabonate ophthalmic gel 0.5 % is an additional formulation option for the short-term treatment of post-operative inflammation and pain in patients who have undergone ocular surgery. It provides uniform dosing of a topical ophthalmic corticosteroid that has been demonstrated to be effective and well-tolerated in the treatment of ocular inflammation.

Similar content being viewed by others
References
El-Harazi SM, Feldman RM. Control of intra-ocular inflammation associated with cataract surgery. Curr Opin Ophthalmol. 2001;12(1):4–8.
Mohammadpour M, Jafarinasab MR, Javadi MA. Outcomes of acute postoperative inflammation after cataract surgery. Eur J Ophthalmol. 2007;17(1):20–8.
Amon M, Busin M. Loteprednol etabonate ophthalmic suspension 0.5%: efficacy and safety for postoperative anti-inflammatory use. Int Ophthalmol. 2012;32(5):507–17.
Comstock TL, Decory HH. Advances in corticosteroid therapy for ocular inflammation: loteprednol etabonate. Int J Inflam. 2012. doi:10.1155/2012/789623.
McGhee CN, Dean S, Danesh-Meyer H. Locally administered ocular corticosteroids: benefits and risks. Drug Saf. 2002;25(1):33–55.
Coffey MJ, Decory HH, Lane SS. Development of a non-settling gel formulation of 0.5% loteprednol etabonate for anti-inflammatory use as an ophthalmic drop. Clin Ophthalmol. 2013;7:299–312.
American Academy of Ophthalmology Cataract and Anterior Segment Panel. Perferred Practice Pattern Guidelines®: cataract in the adult eye. San Francisco: American Academy of Ophthalmology; 2011.
Pavesio CE, Decory HH. Treatment of ocular inflammatory conditions with loteprednol etabonate. Br J Ophthalmol. 2008;92(4):455–9.
Lotemax (loteprednol etabonate ophthalmic suspension 0.5%). US prescribing information. Tampa: Bausch & Lomb Incorporated; 2006.
Lotemax (loteprednol etabonate ophthalmic ointment 0.5%). US prescribing information. Tampa: Bausch & Lomb Incorporated; 2011.
Stewart R, Horwitz B, Howes J, et al. Double-masked, placebo-controlled evaluation of loteprednol etabonate 0.5% for postoperative inflammation. Loteprednol Etabonate Post-operative Inflammation Study Group 1. J Cataract Refract Surg. 1998;24(11):1480–9.
A double-masked, placebo-controlled evaluation of 0.5% loteprednol etabonate in the treatment of postoperative inflammation. The Loteprednol Etabonate Postoperative Inflammation Study Group 2. Ophthalmology. 1998;105(9):1780–6.
Comstock TL, Paterno, Singh A, et al. Safety and efficacy of loteprednol etabonate ophthalmic ointment 0.5% for the treatment of inflammation and pain following cataract surgery. Clin Ophthalmol. 2011;5:177–86.
Asbell P, Howes J. A double-masked, placebo-controlled evaluation of the efficacy and safety of loteprednol etabonate in the treatment of giant papillary conjunctivitis. CLAO J. 1997;23(1):31–6.
Friedlaender MH, Howes J. A double-masked, placebo-controlled evaluation of the efficacy and safety of loteprednol etabonate in the treatment of giant papillary conjunctivitis. The Loteprednol Etabonate Giant Papillary Conjunctivitis Study Group I. Am J Ophthalmol. 1997;123(4):455–64.
Dell SJ, Shulman DG, Lowry GM, et al. A controlled evaluation of the efficacy and safety of loteprednol etabonate in the prophylactic treatment of seasonal allergic conjunctivitis. Loteprednol Allergic Conjunctivitis Study Group. Am J Ophthalmol. 1997;123(6):791–7.
Pflugfelder SC, Maskin SL, Anderson B, et al. A randomized, double-masked, placebo-controlled, multicenter comparison of loteprednol etabonate ophthalmic suspension, 0.5%, and placebo for treatment of keratoconjunctivitis sicca in patients with delayed tear clearance. Am J Ophthalmol. 2004;138(3):444–57.
Controlled evaluation of loteprednol etabonate and prednisolone acetate in the treatment of acute anterior uveitis. Loteprednol Etabonate US Uveitis Study Group. Am J Ophthalmol. 1999;127(5):537–44.
Fiscella RG. Ophthalmic drug formulations. In: Bartlett JD, Jaanus SD, editors. Clinical Ocular Pharmacology. 5th ed. St Louis: Butterworth-Heinemann; 2008. p. 17–37.
Lotemax (loteprednol etabonate ophthalmic gel 0.5%). US prescribing information. Tampa: Bausch & Lomb Incorporated; 2012.
Stahn C, Lowenberg M, Hommes DW, et al. Molecular mechanisms of glucocorticoid action and selective glucocorticoid receptor agonists. Mol Cell Endocrinol. 2007;275(1–2):71–8.
Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids–new mechanisms for old drugs. N Engl J Med. 2005;353(16):1711–23.
Bodor N. Designing safer ophthalmic drugs by soft drug approaches. J Ocul Pharmacol. 1994;10(1):3–15.
Bodor N. Design of novel soft corticosteroids. Curr Probl Dermatol. 1993;21:11–9.
Bodor N, Buchwald P. Ophthalmic drug design based on the metabolic activity of the eye: soft drugs and chemical delivery systems. AAPS J. 2005;7(4):E820–33.
Druzgala P, Hochhaus G, Bodor N. Soft drugs-10. Blanching activity and receptor binding affinity of a new type of glucocorticoid: loteprednol etabonate. J Steroid Biochem Mol Biol. 1991;38:149–54.
Alberth M, Wu WM, Winwood D, et al. Lipophilicity, solubility and permeability of loteprednol etabonate: a novel, soft anti-inflammatory steroid. J Biopharm Sci. 1991;2:115–25.
Fong R, Leitritz M, Siou-Mermet R, et al. Loteprednol etabonate gel 0.5% for postoperative pain and inflammation after cataract surgery: results of a multicenter trial. Clin Ophthalmol. 2012;6:1113–24.
Rajpal RK, Roel L, Siou-Mermet R, et al. Efficacy and safety of loteprednol etabonate 0.5% gel in the treatment of ocular inflammation and pain after cataract surgery. J Cataract Refract Surg. 2013;39(2):158–67.
Mindel JS, Tavitian HO, Smith H Jr, et al. Comparative ocular pressure elevation by medrysone, fluorometholone, and dexamethasone phosphate. Arch Ophthalmol. 1980;98(9):1577–8.
Bhargava AS, Staben P. Effect of corticosteroids on intraocular pressure, ascorbic acid and sialic acid concentrations in aqueous humour and on mucopolysaccharides biosynthesis in the cornea of rabbits. Arzneimittelforschung. 1980;30(7):1123–6.
Cantrill HL, Palmberg PF, Zink HA, et al. Comparison of in vitro potency of corticosteroids with ability to raise intraocular pressure. Am J Ophthalmol. 1975;79(6):1012–7.
Bodor N, Bodor N, Wu WM. A comparison of intraocular pressure elevating activity of loteprednol etabonate and dexamethasone in rabbits. Curr Eye Res. 1992;11(6):525–30.
Wu J-S, Zhong X-W, Zhang X-X, et al. A randomized controlled study on the application of 0.5% loteprednol etabonate eye drops after LASIK surgery. Chin J Exp Ophthalomol. 2012;30(7):641–5.
White EM, Macy JI, Bateman KM, et al. Comparison of the safety and efficacy of loteprednol etabonate 0.5%/tobramycin 0.3% with dexamethasone 0.1%/tobramycin 0.3% in the treatment of blepharokeratoconjunctivitis. Curr Med Res Opin. 2008;24:287–96.
Chen M, Gong L, Sun X, et al. A multicenter, randomized, parallel-group, clinical trial comparing the safety and efficacy of loteprednol etabonate 0.5%/tobramycin 0.3% with dexamethasone 0.1%/tobramycin 0.3% in the treatment of Chinese patients with blepharokeratoconjunctivitis. Curr Med Res Opin. 2012;28(3):1–10.
Holland EJ, Bartlett JD, Paterno MR, et al. Effects of loteprednol/tobramycin versus dexamethasone/tobramycin on intraocular pressure in healthy volunteers. Cornea. 2008;27(1):50–5.
Lane SS, Holland EJ. Loteprednol etabonate 0.5% versus prednisolone acetate 1.0% for the treatment of inflammation after cataract surgery. J Cataract Refract Surg. 2013;39(2):168–73.
Bartlett JD, Horwitz B, Laibovitz R, et al. Intraocular pressure response to loteprednol etabonate in known steroid responders. J Ocul Pharmacol. 1993;9(2):157–65.
Holland EJ, Djalilian AR, Sanderson JP. Attenuation of ocular hypertension with the use of topical loteprednol etabonate 0.5% in steroid responders after corneal transplantation. Cornea. 2009;28(10):1139–43.
Li W-T, Li H, Wei J. Clinical study on the application of 5 g/L loteprednol etabonate ophthalmic suspension after LASIK. Int J Ophthalmol. 2010;10(9):1792–3.
Gao D-H, Li K-J, Lu H. Clinical observations on the effect of lotemax on changes in intraocular pressure after LASEK. Int J Ophthalmol. 2010;10(9):806–7.
Glogowski S, Proksch JW. Ocular and systemic pharmacokinetics of loteprednol etabonate gel (0.5%) following topical ocular administration to rabbits [poster no. D1138]. Annual Meeting of the Association for Research in Vision and Ophthalmology; 6–9 May 2012, Fort Lauderdale.
Aziz R. Pharmacology review(s): New Drug Application Number 202872 (loteprednol etabonate ophthalmic gel, 0.5%). Silver Spring: Center for Drug Evaluation and Research; 2012.
Comstock TL, Siou-Mermet R, Erb T, et al. Efficacy of loteprednol etabonate gel 0.5% in the treatment of inflammation and pain in post-cataract surgery patients [poster no. 39]. Annual Meeting of the American Academy of Optometry; 24-27 Oct 2012, Phoenix.
Rajpal RK, Siou-Mermet R, Erb T. Loteprednol etabonate (LE) gel 0.5% in the treatment of ocular inflammation and pain following cataract surgery [poster no. PO021]. Annual Meeting of the American Academy of Opthalmology; 10–13 Nov 2012, Chicago.
Apt L, Henrick A, Silverman LM. Patient compliance with use of topical ophthalmic corticosteroid suspensions. Am J Ophthalmol. 1979;87(2):210–4.
Disclosure
The preparation of this review was not supported by any external funding. During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on this article. Changes resulting from comments received were made by the author on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Additional information
The manuscript was reviewed by: R.G. Fiscella, Department of Pharmacy Practice, University of Illinois at Chicago, Chicago, IL, USA, R.K. Rajpal, See Clearly Vision Group, McLean, VA, USA.
Rights and permissions
About this article
Cite this article
Lyseng-Williamson, K.A. Loteprednol Etabonate Ophthalmic Gel 0.5 %: A Review of Its Use in Post-Operative Inflammation and Pain Following Ocular Surgery. Drugs 73, 949–958 (2013). https://doi.org/10.1007/s40265-013-0073-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-013-0073-8