Abstract
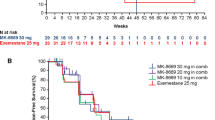
Oral everolimus (Afinitor®) in combination with exemestane is indicated for the treatment of hormone receptor-positive, human epidermal growth factor receptor (HER) 2-negative advanced breast cancer in postmenopausal women after failure of treatment with letrozole or anastrozole (in the USA) or after recurrence of progression following a nonsteroidal aromatase inhibitor (AI) in women without symptomatic visceral disease (in the EU). Everolimus, a selective inhibitor of mammalian target of rapamycin (mTOR), inhibits the downstream signalling events of the mTOR pathway. This review summarizes the pharmacology of everolimus and reviews its efficacy and tolerability when administered in combination with exemestane in postmenopausal women with oestrogen receptor-positive, HER2-negative advanced breast cancer refractory to nonsteroidal AIs. In the well-designed BOLERO-2 study, the addition of everolimus to exemestane was shown to significantly prolong progression-free survival in this patient population. However, treatment-emergent adverse events and treatment discontinuations occurred more frequently with combination therapy than with exemestane alone, suggesting a need for careful benefit/risk assessment prior to initiating therapy. Mature survival data from this study are awaited and additional studies would help to further demonstrate the benefit of combination therapy. Nevertheless, current evidence suggests that everolimus plus exemestane combination therapy may be a useful treatment option in patients with postmenopausal hormone receptor-positive, HER2-negative, advanced breast cancer refractory to nonsteroidal AIs.



Similar content being viewed by others
References
World Health Organization. Breast cancer: prevention and control. 2012. http://www.who.int/cancer/detection/breastcancer/en/index1.html. Accessed 21 Dec 2012.
Rimawi MF, Osborne CK. Breast cancer: blocking both driver and escape pathways improves outcomes. Nat Rev Clin Oncol. 2012;9(3):133–4.
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378(9793):771–84.
Perez EA, Romond EH, Suman VJ, et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol. 2011;29(25):3366–73.
Fedele P, Calvani N, Marino A, et al. Targeted agents to reverse resistance to endocrine therapy in metastatic breast cancer: where are we now and where are we going? Crit Rev Oncol Hematol. 2012;84(2):243–51.
Rugo HS, Keck S. Reversing hormone resistance: have we found the golden key? J Clin Oncol. 2012;30(22):2707–9.
Villarreal-Garza C, Cortes J, Andre F, et al. mTOR inhibitors in the management of hormone receptor-positive breast cancer: the latest evidence and future directions. Ann Oncol. 2012;23(10):2526–35.
Dobashi Y, Watanabe Y, Miwa C, et al. Mammalian target of rapamycin: a central node of complex signaling cascades. Int J Clin Exp Pathol. 2011;4(5):476–95.
Burris HA 3rd. Overcoming acquired resistance to anticancer therapy: focus on the PI3K/AKT/mTOR pathway. Cancer Chemother Pharmacol. 2013. doi:10.1007/s00280-012-2043-3.
Beeram M, Tan QTN, Tekmal RR, et al. Akt-induced endocrine therapy resistance is reversed by inhibition of mTOR signaling. Ann Oncol. 2007;18(8):1323–8.
de Graffenried LA, Friedrichs WE, Russell DH, et al. Inhibition of mTOR activity restores tamoxifen response in breast cancer cells with aberrant Akt activity. Clin Cancer Res. 2004;10(23):8059–67.
European Medicines Agency. Afinitor (everolimus): summary of product characteristics. 2012. http://www.emea.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001038/WC500022814.pdf. Accessed 10 Oct 2012.
Novartis. Afinitor (everolimus): US prescribing information. 2012. http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022334s018lbl.pdf. Accessed 10 Oct 2012.
Novartis. CHMP recommends Novartis drug Afinitor® for EU approval marking major milestone in the treatment of advanced breast cancer [media release]. 2012. http://www.novartis.com/newsroom/media-releases/en/2012/1621353.shtml.
US FDA. FDA approves Afinitor for advanced breast cancer [media release]. 2012. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm312965.htm.
Choi J, Chen J, Schreiber SL, et al. Structure of the FKBP12-rapamycin complex interacting with the binding domain of human FRAP. Science. 1996;273(5272):239–42.
Chen J, Zheng XF, Brown EJ, et al. Identification of an 11-kDa FKBP12-rapamycin-binding domain within the 289-kDa FKBP12-rapamycin-associated protein and characterization of a critical serine residue. Proc Natl Acad Sci USA. 1995;92(11):4947–51.
Mabuchi S, Altomare DA, Cheung M, et al. RAD001 inhibits human ovarian cancer cell proliferation, enhances cisplatin-induced apoptosis, and prolongs survival in an ovarian cancer model. Clin Cancer Res. 2007;13(14):4261–70.
Lane HA, Wood JM, McSheehy PMJ, et al. mTOR inhibitor RAD001 (everolimus) has antiangiogenic/vascular properties distinct from a VEGFR tyrosine kinase inhibitor. Clin Cancer Res. 2009;15(5):1612–22.
O’Reilly T, McSheehy PM, Wartmann M, et al. Evaluation of the mTOR inhibitor, everolimus, in combination with cytotoxic antitumor agents using human tumor models in vitro and in vivo. Anticancer Drugs. 2011;22(1):58–78.
Tabernero J, Rojo F, Calvo E, et al. Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: a phase I tumor pharmacodynamic study in patients with advanced solid tumors. J Clin Oncol. 2008;26(10):1603–10.
O’Donnell A, Faivre S, Burris HA 3rd, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral mammalian target of rapamycin inhibitor everolimus in patients with advanced solid tumors. J Clin Oncol. 2008;26(10):1588–95.
Baselga J, Semiglazov V, van Dam P, et al. Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. J Clin Oncol. 2009;27(16):2630–7.
Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441(7092):424–30.
Treeck O, Wackwitz B, Haus U, et al. Effects of a combined treatment with mTOR inhibitor RAD001 and tamoxifen in vitro on growth and apoptosis of human cancer cells. Gynecol Oncol. 2006;102(2):292–9.
Boulay A, Rudloff J, Ye J, et al. Dual inhibition of mTOR and estrogen receptor signaling in vitro induces cell death in models of breast cancer. Clin Cancer Res. 2005;11(14):5319–28.
Bachelot T, Bourgier C, Cropet C, et al. Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer with prior exposure to aromatase inhibitors: a GINECO study. J Clin Oncol. 2012;30(22):2718–24.
Ellard SL, Clemons M, Gelmon KA, et al. Randomized phase II study comparing two schedules of everolimus in patients with recurrent/metastatic breast cancer: NCIC Clinical Trials Group IND.163. J Clin Oncol. 2009;27(27):4536–41.
Awada A, Cardoso F, Fontaine C, et al. The oral mTOR inhibitor RAD001 (everolimus) in combination with letrozole in patients with advanced breast cancer: results of a phase I study with pharmacokinetics. Eur J Cancer. 2008;44(1):84–91.
Glantschnig H, Fisher JE, Wesolowski G, et al. M-CSF, TNFalpha and RANK ligand promote osteoclast survival by signaling through mTOR/S6 kinase. Cell Death Differ. 2003;10(10):1165–77.
Gnant M, Baselga J, Rugo HS, et al. Effect of everolimus on bone marker levels and progressive disease in bone in BOLERO-2. J Natl Cancer Inst. 2013. doi:10.1093/jnci/djt026.
Diaz-Gonzalez JA, Russell J, Rouzaut A, et al. Targeting hypoxia and angiogenesis through HIF-1alpha inhibition. Cancer Biol Ther. 2005;4(10):1055–62.
Lorusso G, Ruegg C. The tumor microenvironment and its contribution to tumor evolution toward metastasis. Histochem Cell Biol. 2008;130(6):1091–103.
Curran MP. Everolimus: in patients with subependymal giant cell astrocytoma associated with tuberous sclerosis complex. Paediatr Drugs. 2012;14(1):51–60.
Kirchner GI, Meier-Wiedenbach I, Manns MP. Clinical pharmacokinetics of everolimus. Clin Pharmacokinet. 2004;43(2):83–95.
Cheung W, Ligia C, Jappe A. Pharmacokinetic (PK) profile of a single dose of everolimus (10 mg) administered with a low- or high-fat meal and under fasting conditions in healthy subjects [abstract no. e15080]. American Society of Clinical Oncology Annual Meeting, Chicago (IL); 3–7 June 2011.
Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366(6):520–9.
Hortobagyi GN, Piccart M, Rugo H, et al. Everolimus for postmenopausal women with advanced breast cancer: updated results of the BOLERO-2 phase III trial [abstract no. S3–7]. Cancer Res. 2011;71(24 Suppl):S3–7.
Piccart-Gebhart MJ, Noguchi S, Pritchard KI, et al. Everolimus for postmenopausal women with advanced breast cancer: updated results of the BOLERO-2 phase III trial [abstract no. 559 plus poster]. American Society of Clinical Oncology Annual Meeting, Chicago (IL); 1–5 June 2012.
Gnant M, Noguchi S, Ito Y, et al. Safety of everolimus for women over 65 years of age with advanced breast cancer: 18-mo follow-up of BOLERO-2 [abstract no. 351P plus poster]. 37th European Society for Medical Oncology Congress, Vienna; 28 Sep–2 Oct 2012.
Campone M, Noguchi S, Pritchard K, et al. Efficacy and safety of everolimus in postmenopausal women with advanced breast cancer (BOLERO-2): effect of visceral metastases and prior endocrine therapy [abstract no. 324PD plus poster]. 37th European Society for Medical Oncology Congress, Vienna; 28 Sep–2 Oct 2012.
Beck JT, Rugo HS, Burris HA, et al. BOLERO-2: health-related quality of life in metastatic breast cancer patients treated with everolimus and exemestane versus exemestane [abstract no. 539 plus poster]. American Society of Clinical Oncology Annual Meeting, Chicago (IL); 1–5 June 2012.
Burris HA 3rd., Beck JT, Rugo HS, et al. Health-related quality of life (QOL) in advanced breast cancer patients treated with everolimus and exemestane versus exemestane monotherapy [abstract no. 334P plus poster]. 37th European Society for Medical Oncology Congress, Vienna; 28 Sep–2 Oct 2012.
Noguchi S, Masuda N, Iwata H, et al. Efficacy of everolimus with exemestane versus exemestane alone in Asian patients with HER2-negative, hormone-receptor-positive breast cancer in BOLERO-2. Breast Cancer. 2013. doi:10.1007/s12282-013-0444-8.
Iacovelli R, Palazzo A, Mezi S, et al. Incidence and risk of pulmonary toxicity in patients treated with mTOR inhibitors for malignancy: a meta-analysis of published trials. Acta Oncol. 2012;51(7):873–9.
Cardoso F, Costa A, Norton L, et al. 1st International consensus guidelines for advanced breast cancer (ABC 1). Breast. 2012;21(3):242–52.
Buzdar A, Douma J, Davidson N, et al. Phase III, multicenter, double-blind, randomized study of letrozole, an aromatase inhibitor, for advanced breast cancer versus megestrol acetate. J Clin Oncol. 2001;19(14):3357–66.
Buzdar AU, Jonat W, Howell A, On behalf of the Arimidex Study Group, et al. Anastrozole versus megestrol acetate in the treatment of postmenopausal women with advanced breast carcinoma: results of a survival update based on a combined analysis of data from two mature phase III trials. Cancer. 1998;83(6):1142–52.
Chia S, Gradishar W, Mauriac L, et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J Clin Oncol. 2008;26(10):1664–70.
Di Leo A, Jerusalem G, Petruzelka L, et al. Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. J Clin Oncol. 2010;28(30):4594–600.
Novartis Pharmaceuticals. Everolimus in combination with trastuzumab and paclitaxel in the treatment of HER2 positive locally advanced or metastatic breast cancer (BOLERO-1) [ClinicalTrials.gov identifier NCT00876395]. US National Institutes of Health, ClinicalTrials.gov. 2013. http://clinicaltrials.gov/ct2/show/NCT00876395?term=BOLERO+1&rank=1. Accessed 18 Feb 2013.
Novartis Pharmaceuticals. Daily everolimus in combination with trastuzumab and vinorelbine in HER2/Neu positive women with locally advanced or metastatic breast cancer (BOLERO-3) [ClinicalTrials.gov identifier NCT01007942]. US National Institutes of Health, ClinicalTrials.gov. 2012. http://clinicaltrials.gov/show/NCT01007942. Accessed 18 Feb 2013.
Disclosure
The preparation of this review was not supported by any external funding. During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on this article. Changes resulting from comments received were made by the author on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Additional information
The manuscript was reviewed by: A.U. Buzdar, Department of Breast Medical Oncology, MD Anderson Cancer Center, University of Texas, Houston, Texas, USA; D. Cameron, Edinburgh University Cancer Research Centre, Western General Hospital, Edinburgh, UK; A. Fabi, Division of Medical Oncology, Department of Medical Oncology, Regina Elena Cancer Institute, Rome; S. Hurvitz, Department of Medicine, University of California–Los Angeles, Los Angeles, USA.
Rights and permissions
About this article
Cite this article
Dhillon, S. Everolimus in Combination with Exemestane: A Review of its Use in the Treatment of Patients with Postmenopausal Hormone Receptor-Positive, HER2-Negative Advanced Breast Cancer. Drugs 73, 475–485 (2013). https://doi.org/10.1007/s40265-013-0034-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-013-0034-2